Drug Metabolism
Contents
Introduction
Drug metabolism is a detoxification function the human body possesses to defend itself from environment hostility. Ideally, a drug should reach the site of action intact, cure the disease, and leave the body after it completes its mission. However, drug developers often face the dilemma that a potential drug is either metabolized/excreted from the body too fast, that the drug can not reach its therapeutic effect, or too slow, that it stays in the body for a long time, causing side effects.(Drug is a xenobiotic that the normal human body doesn't need.) The study of drug metabolism, therefore, serves primarily two purposes
- To elucidate the function and fate of the drug
- To manipulate the metabolic process of a potential drug.
Metabolism Sites
The liver is the primary site for metabolism. It contains the necessary enzymes for metabolism of drugs and other xenobiotics. These enzymes induce two metabolism pathways:
- Phase I (functionalization reactions). The enzymes involved in Phase I reactions are primarily located in the endoplasmic reticulum of the liver cell, they are called microsomal enzymes Eg. Oxidation and hydrolysis. The Phase I reaction introduces a functional group such as a hydroxyl group onto the molecule, or exposes a preexisting functional group
- Phase II (conjugation/biosynthetic reactions) This involves the introduction of a hydrophilic endogenous species, such as glucuronic acid or sulfate, to the drug molecule. Enzymes involved in phase II reactions are mainly located in the cytosol, except glucuronidation enzyme, which is also a microsomal enzyme.Phase II metabolism Drugs are usually lipophilic substances (Oil-like) so they can pass plasma membranes and reach the site of action. Phase II reaction connects this functional group to the endogenous species such as a glucuronic acid. The modified drug molecule may then be hydrophilic enough to be excreted
- Phase III reactions involves further biotransformation of conjugates.
Drug metabolism is basically a process that introduces hydrophilic functionailities onto the drug molecule to facilitate excretion. When the drug molecule is oxidized, hydrolyzed, or covalently attached to a hydrophilic species, the whole molecule becomes more hydrophilic, and is excreted more easily. Although liver is the primary site for metabolism, virtually all tissue cells have some metabolic activities. Other organs having significant metabolic activities include the gastrointestinal tract, kidneys, and lungs. When a drug is administrated orally, it undergoes metabolism in the GI track and the liver before reaching systemic circulation. This process is called first-pass metabolism. First-pass metabolism limits the oral bioavailability of drugs, sometimes significantly.
Fate of Metabolized drugs
- Drugs are ultimately excreted from the body through various routes.The kidney is the major organ for drug excretion. It excretes hydrophilic drug and drug metabolites through glomerular filtration.Macromolecules such as proteins are retained. Lipophilic drug molecules are not directly excreted from the kidney.Only after they are metabolized into more hydrophilic molecules, can they be excreted through the kidneys into the urine.
- Drugs and their metabolites are also excreted into bile. This is usually mediated by protein transporters. Drugs and their metabolites in bile are eventually released into the intestinal tract. The drugs may be reabsorbed into the body from the intestine. Drug metabolites such as glucuronide conjugates, may be converted back to the parent drug in the intestine through glucuronidase enzyme, and then reabsorbed into systemic circulation. This drug recycling process is called enterohepatic recycling.This process, if extensive, may prolong the half-life of the drug.
- The bile drugs and drug metabolites, if not reabsorbed by intestine, are excreted from the body through feces. Also, a variety of orally administrated drugs are excreted through feces because they are not absorbed through the intestine. Oral bioavailability constitutes a major challenge for drug developers. Other routes of excretion, such as sweat, tears, and saliva, are quantitatively less important. Excretion through breast milk is not important to the mother, but may be of key importance to the baby, because the drug may be toxic to the baby. Pulmonary excretion is important for anesthetic gases and vapor drugs.
Metabolic changes - Mass Shifts
List of mass shifts caused by metabolism of common functional groups.
- Glucuronidation: plus 176 u.
- Sulfation: plus 80 u.
- Oxidation (N-, S-): plus 16 u.
- Hydroxylation (aliphatic, aromatic): plus 16 u (or 32, if two sites).
- Dealkylation: minus the alkyl group: minus 14 u for a methyl group, and 28 u for an ethyl group.
- Hydrolysis: minus R-1 for ester hydrolysis into the acid.Source
Key Parameters
- Maximum drug concentration (Cmax)
- Time to peak concentration Tmax (h)
- Absorption half-life t1/2(a) (h)
- Distribution half-life t1/2 (h)
- Elimination half-life t1/2(ß) (h)
- Area under curve (AUC) µg/ml/h
- Volume of distribution (Vd) mg/litre
- Absorption rate constant Ka (h)
- Distribution rate constant K (h)
- Elimination rate constant Ke (h)
- Mean residual time MRT (h)
- Therapeutic concentration tcp(ther)
- Body clearance CL (h)
Databases
- Drug Metabolism
- IUPAC
- University of Minnesota Biocatalysis/Biodegradation database
- MDL Metabolite
- Accelrys Metabolism Database
- Metabolism and Transport Drug interaction database
- Biofrontier/P450
- METEOR
- META
- MetabolExpert
Sample Example
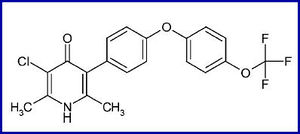
Preclinical drug metabolism and pharmacokinetic evaluation of GW844520
GW844520, a non-chiral 4(1H)-pyridone derivative, is a potent, selective inhibitor of Plasmodium mitochondrial function blocking the electron transport chain machinery. The site of action of this class of compounds is cytochrome b, a critical element of the respiratory complex III or bc1 complex.
Plasmodium falciparum is the species of malaria parasite that predominates in Africa and is the cause of most of the mortality from malaria worldwide. Effective treatment options are often restricted due to drug resistance, particularly for P. falciparum. There is an immediate need to develop novel anti-malarial agents that provide rapid and effective treatment.GW844520 is fully active against isolates carrying resistance determinants to marketed agents and shows no cross-resistance with agents in current use, including atovaquone.
Models/Species:Male CD1 mouse, male Sprague-Dawley rat, male beagle dog, male cynomolgus monkey and mixed gender human liver microsomes.
Test Results
| Pharmacokinetics of GW844520 After Oral Administration to Mouse, Rat, Dog, and Monkey | ||||||
| Species | Dose (mg/kg) | Cmax (µg/mL) | Tmax (h) | AUC0- (µg h/mL) | T1/2 h | F (%) |
| Mouse | 0.5 | 0.28 | 4 | 8.54 | 23.6 | 72 |
| Rat | 0.5 | 0.25 ± 0.02 | 2 (1-2) | 4.14 ± 0.96 | 7.1 ± 1.7a | 118 |
| Dog | 0.4 | 0.14 ± 0.02 | 24 (4-24) | 22.8 ± 7.9 | 214 ± 30 | 51 ± 11 |
| Monkey | 0.4 | 0.12 ± 0.01 | 4 (4-8) | 13.5 ± 0.6 | 88.4 ± 4.4 | 69 ± 6 |
| In Vitro Cell Partitioning of GW844520 in Animal and Human Blood | ||
| Species | Concentration (µg/mL) | Blood to plasma ratio |
| Mouse | 0.25 | 0.56 ± 0.01 |
| 2.5 | 0.48 ± 0.02 | |
| Rat | 0.25 | 0.50 ± 0.02 |
| 2.5 | 0.56 ± 0.03 | |
| Dog | 0.25 | 0.52 ± 0.01 |
| 2.5 | 0.52 ± 0.01 | |
| Monkey | 0.25 | 0.69 ± 0.06 |
| 2.5 | 0.62 ± 0.01 | |
| Human | 0.5 | 0.54 ± 0.06 |
| 5 | 0.60 ± 0.03 | |
| A)Intrinsic clearance CLi, mL/min/g liver | |||||
| Mouse | Rat | Dog | Monkey | Human | |
| Microsomes | 0.7 | <0.5 | <0.5 | <0.5 | <0.5 |
| Hepatocytes | <0.5 | 3.7 | 1.7 | <0.5 | <0.5 |
| B)CYP450 Inhibition IC50 µM | |||||
| 3A4 | 1A2 | 2D6 | 2C9 | 2C19 | |
| >33 | >33 | 3.0 ± 1.8 | >33 | >33 | |