Environment Friendly Technology to Replace Cadmium Coatings
Contents
Introduction
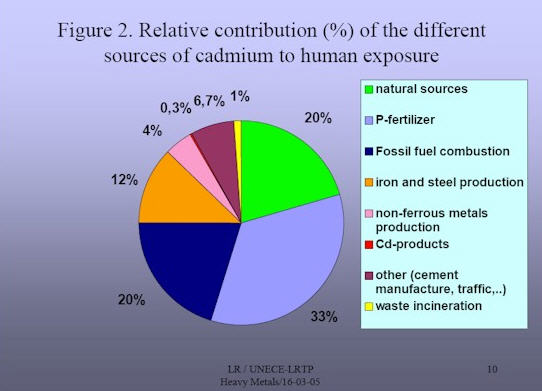
Cadmium is a naturally occurring minor element, one of the metallic components in the earth’s crust and oceans, and present everywhere in our environment. It was first discovered in Germany in 1817 as a by-product of the zinc refining process. Its name is derived from the Latin cadmia and the Greek kadmeia.
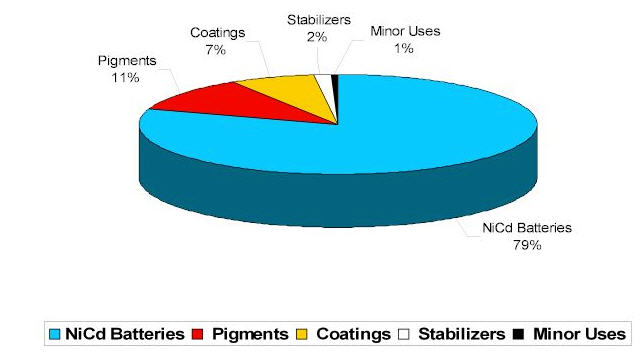
Industrial applications for cadmium were developed in the late 19th and early 20th Century. Cadmium-sulfide based pigments were used as early as 1850 and appeared prominently in the paintings of Vincent Van Gogh in the late 1800s. Thomas A. Edison in the United States and Waldemar Junger in Sweden developed the first nickel-cadmium batteries early in the 20th Century. However, the most significant early use of cadmium was as a corrosion-protection coating on steel.
Cadmium exposure to Humans
- Cadmium Intake From Occupational Exposure
Up to thel960s, very elevated cadmium in air exposure levels were measured in some workplaces, sometimes as high as 1 MOJM3. Since that time, workplace exposures and standards have decreased markedly so that most occupational exposure standards today are in the range from 2 to 50 Ug/M3.In cases where cadmium air levels are higher, the use of personal protective equipment is obligatory. Extensive preventative hygiene programs and medical follow-up programs have been developed to control the risk related to cadmium exposure at the workplace (ACGIH 1996, OSHA 1992, Lauwerys 1986, Cadmium Council 1986). Considering present levels of occupational exposure cadmium intake, use of cadmuim in Workplaces is a matter of concern.
Areas Of Application
Regulatory Concerns
The Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment 2002/95/EC (commonly referred to as the Restriction of Hazardous Substances Directive or RoHS) was adopted in February 2003 by the European Union.The RoHS directive took effect on 1 July 2006, and is required to be enforced and become law in each member state. This directive restricts the use of six hazardous materials in the manufacture of various types of electronic and electrical equipment. It is closely linked with the Waste Electrical and Electronic Equipment Directive (WEEE) 2002/96/EC which sets collection, recycling and recovery targets for electrical goods and is part of a legislative initiative to solve the problem of huge amounts of toxic e-waste.
- It restricts the use of the following six substances:
1. Lead (Pb)
2. Mercury (Hg)
3. Cadmium (Cd)
4. Hexavalent chromium (Cr6+)
5. Polybrominated biphenyls (PBB)
6. Polybrominated diphenyl ether (PBDE)
Thus,Is id evident that cadmium is Losing its place in Industrial Usage.Thus, it becomes imperative that there is a need for finding out alternative technology can can replace cadmium and provide similar features which will be more environment friendly.
- Other Issues Related to Use of Cadmium.
1. Cadmium is a toxin for both animals and plants.
2. Cadmium is known to sublimate in a hard vacuum environment .The sublimation products, which are conductive, can redeposit resulting in short circuits. The sublimation products may also interfere with sensitive optics.
3. Cyanide solutions used in Cadmium plating are highly Toxic.
4. Cadmium is subject to the spontaneous growth of Cadmium whiskers. Cadmium whiskers (like tin whiskers) grow spontaneously and are capable of causing electrical failures ranging from parametric deviations to sustained plasma arcing that can result in catastrophic short circuits.
Rationale
- It is very difficult to ensure complete protection of steel from corrosive environment at temperatures as high as 900°F.
- Traditionally used Ni-Cd Coatings involve use of Hexavalent chromium, cadmium and cyanides which are very toxic. Thus, making it difficult to ensure Safely of the work environment.
Solution to the Problem
Patent Portfolio
- US6869690B1 :- This patent shows how Ni-Zn can be used to replace NI-Cd for Corrosion/Heat resistance.
- US7018486B2 :-This patent Proposes Use of environment friendly Trivalent Chromium Instead of hazardous hexavalent Chromium to enhance the anti-corrosive strength if the coating.
- US6757134B2 :-This patent explains a method of forming a thin film of high density on the substrate in order to remove any voids or grooves which might initiate corrosion.
Benefits
- Environmentally friendly alternative to NiCd.
- Eliminates Materials of Concern from the product such as Cadmium, hexavalent chromium and Cyanide from the plating process.
- Provides sacrificial Protection to the steel also as in case of cadmium.
- Offers equivalent or superior protection to the baseline NiCd coatings.
- Chemicals used in cadmium and cyanide processes are toxic to humans and are strictly regulated by local, state, and federal agencies. Replacing those processes with a non-cadmium/cyanide process reduces the volume of hazardous waste, and decreases the amount of cadmium and cyanide at the facility. The reduction of hazardous waste helps facilities meet the requirements of waste reduction under RCRA, 40 CFR 262, Appendix. It may also help facilities reduce their generator status and lessen the amount of regulations (i.e., recordkeeping, reporting, inspections, transportation, accumulation time, emergency prevention and preparedness, and emergency response) they are required to comply with under RCRA, 40 CFR 262. In addition, this technology allows facilities to eliminate cadmium and cyanide bath solutions, so there is less chance that the facility would exceed reporting thresholds for cadmium and cyanide under SARA Title III (40 CFR 300, 355, 370, and 372; and EO 12856). Both process types may require an industrial wastewater discharge permit (local issue).
Application Areas
- Coating material for steel surfaces and certain other metals like aluminum and copper which might be exposed to highly corrosive atmosphere and extremely high temperatures.
Electrical Transmission Industry
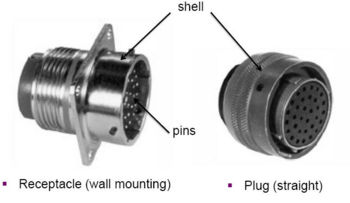
The elimination of cadmium on electrical connectors presents a unique problem not applicable to other components. Items such as fasteners, washers, etc. use cadmium primarily for its corrosion resistance and lubricity. Although these properties are equally important on electrical connectors, the connector shell materials must also provide electromagnetic and radio frequency interference (EMI/RFI) protection. This protection is gained by maintaining an electrically conductive pathway across the connector interface. Suitable alternatives for cadmium must have similar corrosion resistance, lubricity and electrical conductivity.
Fasteners
Cadmium plating is banned as far as fasteners are concerned,you need to take special permission and abide to local norms of effluent treatment for cadmium plating to be practiced.Zinc Alloy plating is of great help-Zinc/Nickel deposits from these baths, with the appropriate chromate conversion coating can withstand well over a thousand hours of Neutral Salt Fog testing.
Automotive Parts
There is an impending EU restriction on lead, cadmuim, hexavalent chrome, and mercury in vehicles.This EU restriction affects vehicles globally since car manufacturers will have to change all components if some fall under this guideline. All major auto manufacturers are now coming up with guidelines and specifications for this transition.