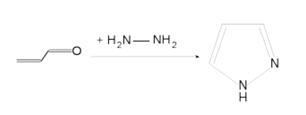Alopecia - Hair Loss
Contents
- 1 Rationale
- 2 Alopecia IPMap
- 3 Introduction
- 4 Goals
- 5 Approach
- 6 IP activity over years
- 7 Major Players
- 8 Anti-androgens
- 9 Minoxidil
- 10 Double action (Anti-androgen + Minoxidil)
- 11 Hair matrix cell activator
- 12 Sebum Production Inhibitor
- 13 Composition nature matrix
- 14 Focus of patents
- 15 Distribution of patents
- 16 Distribution of patents based on different aspects
- 17 Pyrazole compounds
- 18 Inhibition of 5- - reductase by Finasteride
- 19 Questions Dolcera Answers
- 20 New Combinations based on IP study?
- 21 IP studies provides
- 22 Conclusions
- 23 Useful links
- 24 Summary
Rationale
- “Medication for men plagued by hair loss has become a topic of interest in Japan since a drug company began marketing it at the end of last year." March 5th, 2006 – [1]
- “An increasing number of companies are apparently turning the Chinese fear of a bald spot into big bucks with some doing so well they are branching out into other countries.” February 16, 2006 – [2]
Alopecia IPMap
Introduction
Hair Basics
- Hair is a complex and delicate part of the body
- Keeping it healthy and beautiful is a challenge
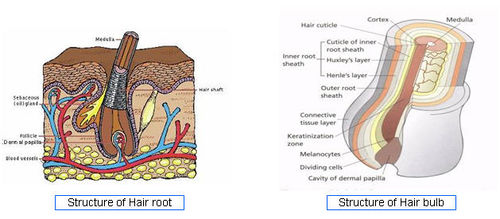
- Structure of Hair root - [3]
- Structure of Hair bulb - [4]
Reasons for Hair loss
Both men and women lose hair for similar reasons. Hair loss in men is often more dramatic, and follows a specific pattern of loss which has been termed “Male Pattern Baldness” (Androgenetic Alopecia).
Main reasons
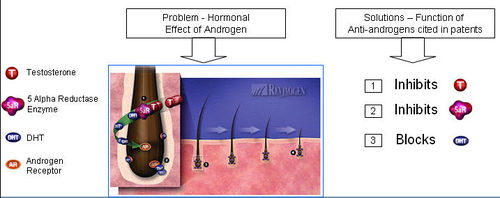
- Hormonal effect of androgen
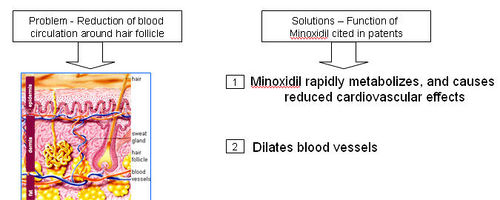
- Reduction of blood circulation around hair follicle
- Deactivation of hair matrix cells
Some facts from Japan
- Market size: ¥ 30 Billion
- Number of products: more than 100
(JICST-EPlus - Japanese Science & Technology)
Goals
The goal of this report is to:
- Summarize IP activity over the years
- Identify major players
- Conduct patent analysis
a) Composition b) Nature c) Action
And then
- Analyze patents pertaining to high sebum activity
Approach
- A broad search was conducted on hair loss patents.
- Patent information was sourced through SIP.
- A set of patents was selected for analysis.
Composition of treatment for causes are identified and categorized as follows:
- Anti-androgen
- Minoxidil
- Double action (Anti-androgen + Mindoxidil)
- Hair matrix cells activator
- Sebum production inhibitor
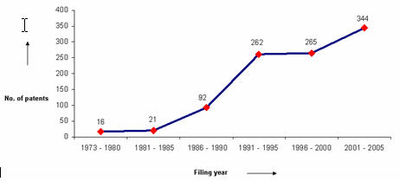
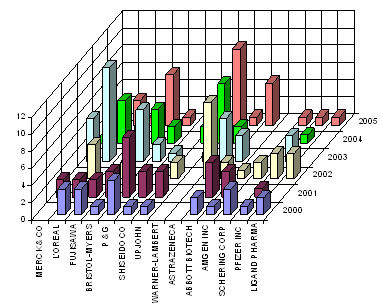
IP activity over years
The graph indicates:
- Number of patents filed every 5 years (except for first 7 years).
- First solution proposed in 1973
- Filing trend indicates steep rise in activity recently.
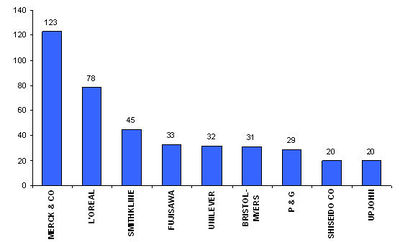
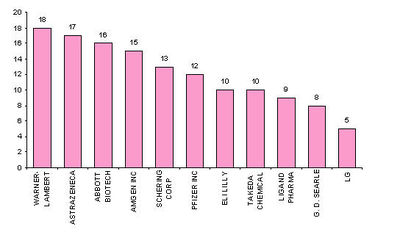
Major Players
- Active Assignees
Assignees currently active with more than 5 patents to their credit during 2000-2005.
- Warner with 9 patents,
- Bristol with 6 and
- Abbott with 5.
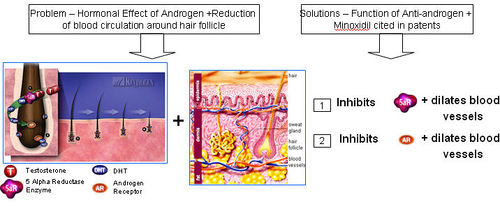
Anti-androgens
- Anti-androgen is a substance that inhibits biological effects of androgenic hormones
- 5-alpha reductase + Testosterone = Dihydrotestosterone (DHT)
- DHT attaches to an Androgen Receptor.
- DHT causes increase in hair loss and gradual miniaturization of the follicle, which eventually dies resulting hair loss
=== Functions of Anti-androgen === Anti-androgen
IP Map for Anti-androgen
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20060009430
BLOTECH (2004) |
Natural extracts | Palmetto berry extract (fatty acids & sterols), Pumpkin seed extract (Vitamins-B, alpha-linolenic acid, amino acids and phytosterols), Quercetin (Flavonoids) and Beta-sitosterol (Rice bran, wheat germ, corn oils and soybeans) | Fatty acids – Inhibit testosterone
Sterols - Mechanism of action unknown. Quercetin results in cell growth cycle. Beta-sitosterol reduce inflammation on scalp |
| US20060009427
WARNER LAMBERT(2004) |
Organic compound | New class of 4-cycloalkoxy benzonitrile derivatives and salts | Acts as androgen receptor modulators and blocks formation of DHT. |
| US20050085467
WARNER LAMBERT(2004) |
Organic compound | New class of 6-sulfonamido-quinolin-2-one and 6-sulfonamido-2-oxo-chromene derivatives. | The compounds inhibit, or decrease, activation of androgen receptor by androgens. |
| US20050118282
APHIOS Corp (2003) |
Natural extracts | Supercritical fluid isolate of Saw Palmetto and Sperol (Serenoa repens berry) and their analogs or derivatives. | Modulates androgenic activity by inhibiting 5.alpha.-reductase activity. |
| US20060009429
Fundacion Pablo Cassara (2003) |
Nucleotide | Pharmacologically active oligonucleotides (encompass both DNA and S-DNA bond) | Oligonucleotides inhibit androgen receptor (AR) expression at very low concentrations in skin and hair follicle |
| US20030007941
PFIZER INC (2001) |
Organic compound | Thyromimetic compounds (structurally similar to thyronine) with finasteride, or cyproterone acetate | Activates thyroid hormone receptors in hair follicle which in turn promote elasticisation of follicle walls and hair follicle |
| US20030073616
N/A (1995) |
Peptides/nucleic acid | Bradykinin antagonist (peptide of plasma origin from kininogen precursor-kallikrein) | Inhibit synthesis of bradykinin receptors or compounds by binding to B2 receptor |
| EP0279010
KAO Corp (1987) |
Natural extracts | Walnut extract (leaves/pericarps) with an organic solvent | Blocks formation of DHT |
Minoxidil
- A thick network of tiny veins and arteries lines the outer wall of the follicle. Blood pumps through the bulb and hair via this network
- Minoxidil dilates blood vessels; which is also called as “potassium channel opener”
- Minoxidil sulfate (MS) appears to be the active metabolite responsible for hair growth stimulation.
=== Functions of Monoxidil === Minoxidil
IP Map for Minoxidil
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20040157856
WARNER LAMBERT(2002) |
Organic compound | Benzopyran compounds | Rapidly metabolizes, and causes reduced cardiovascular effects as compared to other known potassium channel openers |
| US20050053572
LG HOUSEHOLD & HEALTH CARE(2001) |
Natural extracts | Sophora flavescens extract (alkaloids & flavonoids, luteolin-7-glucose and cytosine) Hinokitiol (Taiwan hinoki oil, Aomori, Western Red Cedar oil) and Nicotinamide (Vitamin B complex) | Promotes function of cell activity and dilates blood vessels |
Double action (Anti-androgen + Minoxidil)
- Combination of Minoxidil + Anti-androgen (double action) composition for effective treatment of Male-Pattern Baldness
=== Functions of (Anti-androgen + Minoxidil) === Anti-androgen and Minoxidil
IP Map for (Anti-androgen + Minoxidil)
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20060052405
N/A(2000) |
Peptides | Testosterone blocker or vascular toner (Flutamide, cyproterone acetate, spironolactone, progesterone, or analogs or derivatives) and minoxidil mixed along with non-retinoid penetration enhance and sunscreen | Inhibits 5.alpha.-reductase activity (block DHT) and increase blood flow on the scalp |
| US20050123577
L'OREAL(2000) |
Peptides | Prostaglandin (polyunsaturated fatty acids) EP-2, EP-3 EP-4 receptor agonist with Minoxidil, 2,4-diaminopyrimidine 3-oxide, and Aminexil, cyclic AMP | Minoxidil (designed to mimic nitric oxide's effects) grows hair via prostaglandin-H synthase stimulation. EP-3 and EP-4 are expressed in anagen hair follicles which induce a reduction in the level of cAMP |
| US6447762
COLOMER GROUP(1999) |
Natural extract | Hop extract (oil contains terpenes and humulene), Rosemary extract (hydroalcohol), Swertia extract (glycol with a swertiamarin), Silanodiol salicylate (biologically active silicon compound) | Inhibits activity of 5-alpha-reductase, protects follicular cell membranes by neutralizing action of oxidation reaction in tissues, stimulates hair follicles and blood circulation to the hair root, supplies oxygen and nutrients to base of follicle, retains humidity, avoids dehydration of scalp |
Hair matrix cell activator
- Stem cells of the hair follicle are gathered in the basal layer of the outer root sheath bulge.
- It is from these cells that matrix cells are formed.
- Growth and differentiation of the matrix cells are under the influence of substances produced by cells of the dermal papilla.
=== Functions of Hair matrix cell activator === Hair matrix cell activator
IP Map for Hair matrix cell activator
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20020052498
SHISEIDO(1999) |
Organic compound | (2-substituted oxyphenyl) alkanamide derivative and its salt | Mechanism of action has not been made clear, having excellent hair follicle activating action and regrowth promoting effect |
| US20040071647
L'OREAL(1998) |
Peptides | Metalloprotease (MMP-9) inhibitor (thiol or a hydroxamate) other than chelating calcium ions | Reducing the expression of MMPs (Metalloproteases) in the scalp - slow down or inhibit the degradation of the perifollicular matrix (extracellular matrix surround the hair follicle) |
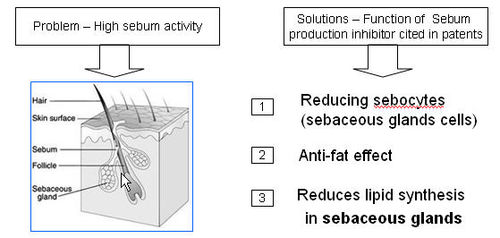
Sebum Production Inhibitor
- Sebum, a complex mixture of lipid substances, is secreted from sebaceous glands associated with hair follicles.
- The inhibitor blocks the excessive sebum production produces greasy effect on hair and scalp and also responsible for thinning and loosing of hair.
Functions of Sebum Production Inhibitor
IP map for Sebum Production Inhibitor
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20050277699
Unilever(2005) |
Natural extract and organic compound | Polyamine (putrescine, spermine or spermidine) analogs and/or derivatives; DFMO; N-acetyl cysteines; neutralized salts of a non-hydroxy C2-C40 dicarboxylic acids, preferably malonate salts; and mixtures thereof. | Decreasing sebum production and/or pore size |
| US20050244362
KAO COPR.(2004) |
Natural extract and organic compound | Avocado oil (Butyl esters of fatty acids) | Reduce sebum of the hair and scalp |
| US4529587
Unilever(1982) |
organic compound | Biotin antagonist or a salt thereof | Decrease activity of the enzyme acetyl-SCoA-carboxylase and hence reduce lipid synthesis in sebaceous glands so that less sebum is produced |
Composition nature matrix
IP map for Composition nature matrix
| Year | Organic Compound | Natural extracts | Peptides | Nucleotides | Natural extract + Organic comp |
|---|---|---|---|---|---|
| 2005 | .... | .... | .... | .... | UNILEVER (1) |
| 2004 | WARNER (1) | BLOTECH (1) | .... | .... | KAO (1) |
| 2003 | WARNER (1) | APHIOS (1) | .... | FUNDIACION (1) | .... |
| 2002 | WARNER (1) | .... | .... | .... | .... |
| 2001 | PFIZER (1) | LG HEALTH-CARE (1) | .... | .... | .... |
| 2000 | .... | .... | L’OREAL (1) / N/A (1) | .... | .... |
| 1999 | SHISEDIO (1) | COLOMER (1) | .... | .... | .... |
| 1998 | .... | .... | L’OREAL (1) | .... | .... |
| 1995 | .... | .... | N/A (1) | .... | .... |
| 1987 | .... | KAO (1) | .... | .... | .... |
| 1982 | UNILEVER (1) | .... | .... | .... | .... |
Focus of patents
| Focus of patents | Patent no. | Rec. no. |
|---|---|---|
| 2-substituted oxyphenyl alkanamide derivative having excellent hair growth effect. | US20020052498 | 1 |
| Thyromimetic compounds, and its role in treating hair loss | US20030007941 | 2 |
| Saw Palmetto berry extract, pumpkin seed extract, sitosterol and quercetin for the treatment and prevention of the biologically detrimental effects of DHT | US20060009430 | 3 |
| 4-cycloalkoxy benzonitriles and its use as androgen receptor modulators | US20060009427 | 4 |
| Supercritical fluid isolate of Saw Palmetto, Sperol for inhibition of 5-.alpha.-reductase activity | US20050118282 | 5 |
| New class of quinolin-2-ones and chromen-2-ones andtheir use as androgen receptor antagonists | US20050085467 | 6 |
| Antiandrogen oligonucleotides usable for the treatment of dermatological androgen-related disorders | US20060009429 | 7 |
| Bradykinin antagonists for stimulating or inducing hair growth and/or arresting hair loss | US20030073616 | 8 |
| Extract from walnut leaves and/or pericarps as 5 alpha -reductase inhibitor | EP0279010 | 9 |
| Stimulating hair growth using benzopyrans | US20040157856 | 10 |
| Sophora flavescens extract, Coicis semen extract, clove extract, etc for promoting hair growth, function of cell activity and dilating peripheral blood vessels. | US20050053572 | 11 |
| Compositions to prevent or reduce hair loss | US20060052405 | 12 |
| Prostaglandin EP-3 receptor antagonists for reducing hair loss | US20050123577 | 13 |
| Synergic effect arising from the interaction of active ingredients, consisting of three plant extracts and a synthetic organosilicic compound for prevent hair loss and stimulate hair growth | US6447762 | 14 |
| Metalloprotease inhibitors to induce and/or stimulate the growth | US20040071647 | 15 |
| Method of decreasing sebum production and pore size | US20050277699 | 16 |
| Method for reducing sebum on the hair and skin | US4529587 | 17 |
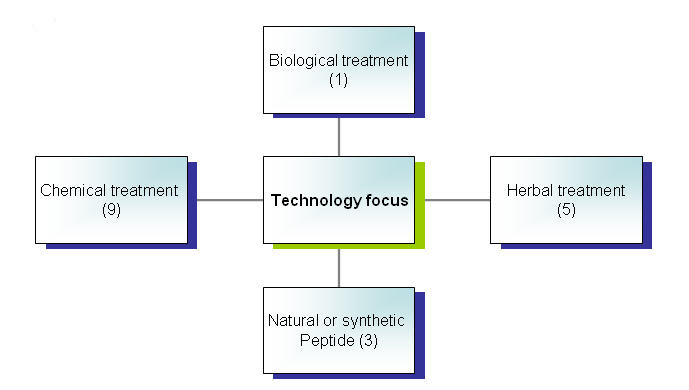
Focus of patents by technology
Distribution of patents
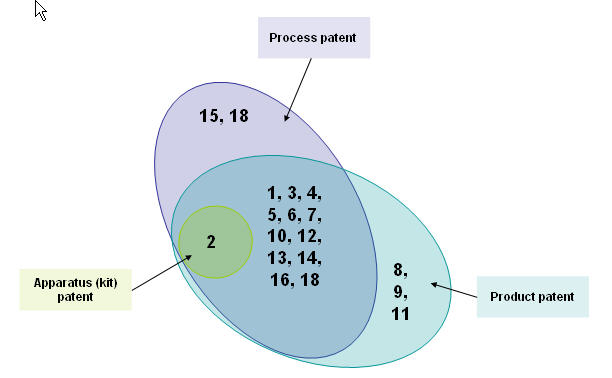
Distribution based on patent types
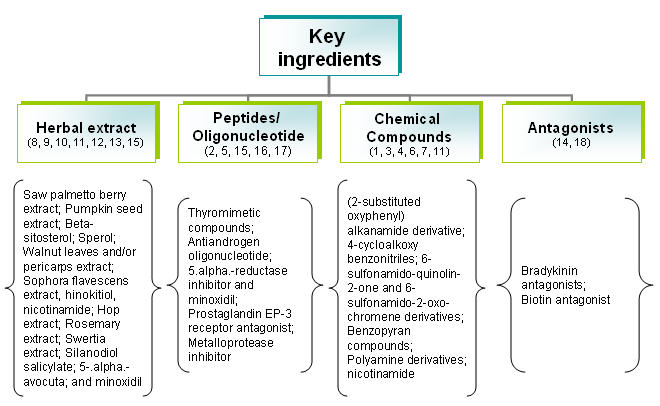
Distribution of patents by their key ingredients
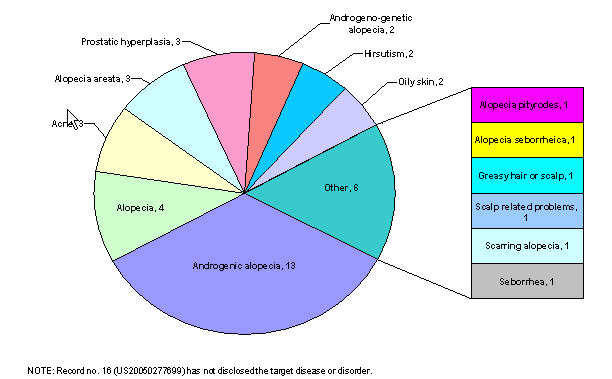
Distribution based on target diseases
Key ingredients vs. Target disease/disorder
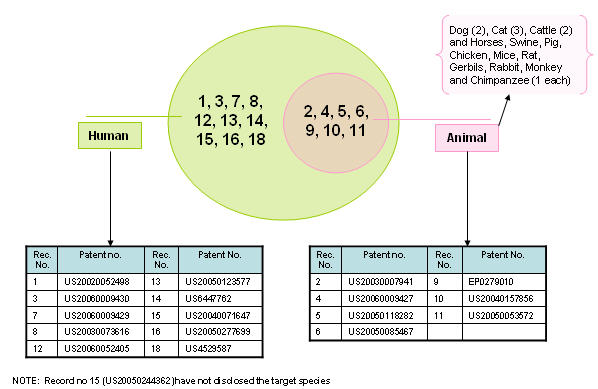
Target species
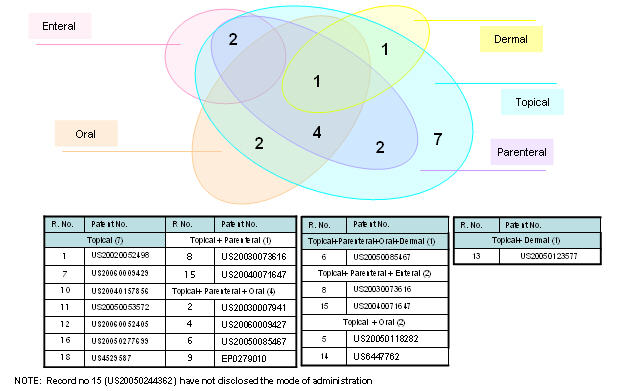
Mode of administration
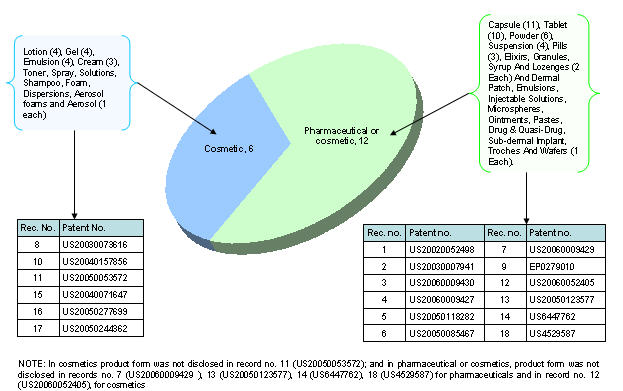
Product type vs. Product form
Distribution of patents based on different aspects
List of patents
| Rec. no. | Patent no. | Rec. no. | Patent no. |
|---|---|---|---|
| 1 | US20020052498 | 10 | US20040157856 |
| 2 | US20030007941 | 11 | US20050053572 |
| 3 | US20060009430 | 12 | US20060052405 |
| 4 | US20060009427 | 13 | US20050123577 |
| 5 | US20050118282 | 14 | US6447762 |
| 6 | US20050085467 | 15 | US20040071647 |
| 7 | US20060009429 | 16 | US20050277699 |
| 8 | US20030073616 | 17 | US20050244362 |
| 9 | EP0279010 | 18 | US4529587 |
Distribution of patents based on target diseases
| Target disease/ disorder | Patent no. | Rec. no. |
|---|---|---|
| Alopecia areata, alopecia pityrodes or alopecia seborrheica, or androgenic alopecia (i.e. male pattern baldness) | US20020052498 | 1 |
| Alopecia areata, male pattern baldness and female pattern baldness | US20030007941 | 2 |
| Androgenic alopecia (i.e. male pattern baldness), prostatic hyperplasia or both. | US20060009430 | 3 |
| Inappropriate activation of the androgen receptor, acne, oily skin, alopecia | US20060009427 | 4 |
| Prostatic hyperplasia, prostatic cancer, hirsutism, acne, male pattern baldness, seborrhea, and other diseases related to androgen hyperactivity | US20050118282 | 5 |
| Alopecia, acne, oily skin, prostrate cancer, hirsutism, and benign prostate hyperplasia | US20050085467 | 6 |
| Androgen-associated hair loss and androgen-skin related disorders. | US20060009429 | 7 |
| Androgenetic or androgenic alopecia or androgeno-genetic alopecia | US20030073616 | 8 |
| Diseases caused by testosterone (male-pattern alopecia) | EP0279010 | 9 |
| Alopecia areata, female pattern hair loss, hair loss secondary to chemotherapy or radiation treatment, stress-related hair loss, self-induced hair loss, scarring alopecia, and alopecia in non-human mammal | US20040157856 | 10 |
| Male pattern alopecia | US20050053572 | 11 |
| Alopecia, androgenic alopecia | US20060052405 | 12 |
| Hair loss | US20050123577 | 13 |
| Male pattern alopecia | US6447762 | 14 |
| Androgenetic, androgenic or androgenogenetic alopecia | US20040071647 | 15 |
| Curing other scalp related problems | US20050244362 | 16 |
Distribution of patents based on application
Interactive Signaling Pathway and linkages
== Players of Wnt inhibition Pathway ==| Patent no. | Key compound | Players of inhibition |
|---|---|---|
| US6664247 | Pyrazole compounds | GSK3 |
| US6989385 | Pyrazole compounds | GSK3 |
| WO2005012256 | Pyrazole compounds | CDK,GSK3 |
| US6974819 | Pyrimidine derivative | GSK3 |
| US6743791 | Heterocyclic compounds | AKT3, GSK-3, ERK2 |
| US20050277773 | Pyrrolo[3,2-d]pyrimidine derivatives | GSK3 |
| US20040072836 | Aza-oxindole derivatives | GSK3, AKT, PKC |
| EP1477489 | Pyrrolopyrimidine derivatives | GSK3 |
| WO0056710 | 3-(Anilinomethylene) oxindoles | GSK3, AKT, PKC |
| WO2003011287 | Pyrazolon derivatives | GSK3, β-catenin |
| US6924141 | Lithium chloride, Wnt3/4/ 7 | β-catenin, GSK3, Wnt |
| US6706685 | Peptide sequence | β-catenin |
| US6683048 | Peptide sequence | α-catenin, β-catenin |
| US6677116 | Peptide sequence LXXLL | β-catenin |
| US6303576 | Peptide sequence LXXLL | β-catenin |
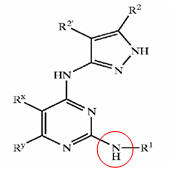
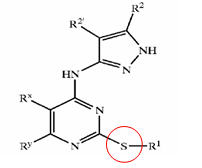
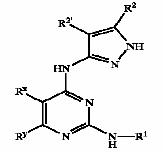
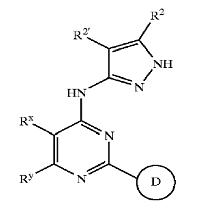
Pyrazole compounds
- Pyrazole (C3H4N2) refers both to the class of simple aromatic ring organic compounds of the heterocyclic series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions and to the unsubstituted parent compound. Being so composed and having pharmacological effects on humans, they are classified as alkaloids although they are not known to occur in nature.
- Pyrazoles are produced synthetically through the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation
- Pyrazoles are used for their analgesic, anti-inflammatory, antipyretic, antiarrhythmic, tranquilizing, muscle relaxing, psychoanaleptic, anticonvulsant, monoamineoxidase inhibiting, antidiabetic and antibacterial activities.
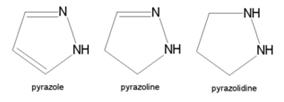
- Structurally related compounds are pyrazoline and pyrazolidine.
GSK3 inhibition by pyrazole compounds
| US6989385 | US6664247 | WO2005012256 |
|---|---|---|
| R1=T-Ring D, wherein
T is a valence bond and Ring D = 5-6 membered aryl or heteroaryl ring; R2 = hydrogen or C1-4 aliphatic and R2'= hydrogen; R3 = -R, -OR, or -N(R4)2, wherein R = hydrogen, C1-6 aliphatic, 5-6 membered heterocyclyl, phenyl, or 5-6 membered heteroaryl, and L is -O-, -S-, or -NH-; and Ring D is substituted by up to three substituents selected from -halo, -CN, -NO2, -N(R4)2, optionally substituted C1-6 aliphatic group, -OR, -C(O)R, -CO2R, -CONH(R<4>), -N(R4)COR, -N(R4)CO2R, -SO2N(R4)2, -N(R4)SO2R, -N(R6)COCH2N(R4)2, -N(R6)COCH2CH2N(R4)2, or -N(R6)COCH2CH2CH2N(R4)2, wherein R = hydrogen, C1-6 aliphatic, phenyl, 5-6 membered heteroaryl ring, or 5-6 membered heterocyclic ring |
X = R1-A-NR4- or a 5- or 6-membered carbocyclic or heterocyclic ring; A is a bond, S02, C=O, NRg(C=O) or O(C=O) wherein Rg is hydrogen or C1-4 hydrocarbyl optionally substituted by hydroxy or C1-4 alkoxy; Y is a bond or an alkylene chain of 1, 2 or 3 carbon atoms in length;
R1 is hydrogen; carbocyclic or heterocyclic group having from 3 to 12 ring members; or C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from halogen (e.g. fluorine), hydroxy, C1-4 hydrocarbyloxy, amino, mono- or di-C1-4 hydrocarbylamino, and carbocyclic or heterocyclic groups having from 3 to 12 ring members, and wherein 1 or 2 of the carbon atoms of the hydrocarbyl group may optionally be replaced by an atom or group selected from 0, S, NH, SO, S02; R2 is hydrogen; halogen; C1-4 alkoxy (e.g. methoxy); or a C1-4 hydrocarbyl group optionally substituted by halogen (e.g. fluorine), hydroxyl or C1-4 alkoxy (e.g. methoxy); R3 is selected from hydrogen and carbocyclic and heterocyclic groups having from 3 to 12 ring members; and R4 is hydrogen or a C1-4 hydrocarbyl group optionally substituted by halogen (e.g. fluorine), hydroxyl or C1-4 alkoxy (e.g. methoxy). |
X is a groupR1-A-NR4-or a 5-or 6-membered carbocyclic or heterocyclic ring;
A is a bond,SO2, C=O, NRg (C=O) or O(C=O) wherein Rg is hydrogen orC14 hydrocarbyl optionally substituted by hydroxy or C1-4 alkoxy;Y is a bond or an alkylene chain of 1,2 or 3 carbon atoms in length;R'is hydrogen; a carbocyclic or heterocyclic group having from 3 to 12 ring members; or a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from halogen (e. g. fluorine), hydroxy, C1-4 hydrocarbyloxy, amino, mono-ordi-Cl 4 hydrocarbylamino, and carbocyclic or heterocyclic groups having from 3 to 12 ring members, and wherein 1 or 2 of the carbon atoms of the hydrocarbyl group may optionally be replaced by an atom or group selected fromO, S, NH, SO, SO2 ;R2 is hydrogen; halogen;C14 alkoxy (e. g. methoxy); or aC14 hydrocarbyl group optionally substituted by halogen (e. g. fluorine), hydroxyl orC14 alkoxy (e. g. methoxy);R3 is selected from hydrogen and carbocyclic and heterocyclic groups having from 3 to 12 ring members; andR4 is hydrogen or a C1-4 hydrocarbyl group optionally substituted by halogen (e. g. fluorine), hydroxyl or C1-4 alkoxy (e. g. methoxy). |
GSK3 inhibition by pryazole pyrimidine amine derivatives
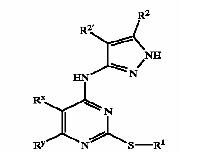
Patent Number: US6989385 Applicant: Vertex Pharmaceuticals Incorporated Title: Pyrazole compounds useful as protein kinase inhibitors Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in the patent:
- [6-(2,6-Dimethylphenyl)-2-(naphthalen-2-ylsulfanyl)-pyrimidin-4-yl]-(5-met- hyl-2H-pyrazol-3-yl)-amine
- [6-(2-Methylphenyl)-2-(naphthalen-2-ylsulfanyl)-pyrimidin-4-yl]-(5-methyl-- 2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenylsulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-methyl-2H-pyra- zol-3-yl)-amine
- [2-(4-Isobutyrylylamino-phenylsulfanyl)-6-phenylpyrimidin-4-yl]-(5-methyl-- 2H-pyrazol-3-yl)-
- [6-(4-Methylpiperazin-1-yl)-2-methylsulfanyl-pyrimidin-4-yl]-(5-methyl-2H-- pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-[6-phenyl-2-(4-propionylamino-phenylsulfanyl)-p- yrimidin-4-yl]-amine
- [2-(4-Cyclopropanecarbonylamino-phenylsulfanyl)-6-phenylpyrimidin-4-yl]-(5- -methyl-2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-{6-phenyl-2-[4-(propane-1-sulfonylamino)-phenyl- sulfanyl]-pyrimidin-4-yl}-amine
- [2-(4-Ethanesulfonylamino-phenylsulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-meth- yl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamidophenyl-sulfanyl)-6-(2-methylphenyl)-pyrimidin-4-yl]-(5-meth- yl-2H-pyrazol-3-yl)-amine
- [2-(4-Isobutanecarbonylamino-phenyl-sulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-- methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-5-methyl-6-phenyl-pyrimidin-4-yl]-(5-meth- yl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-6-(4-methoxyphenyl)-pyrimidin-4-yl]-(5-me- thyl-2H-pyrazol-3-yl)-amine
- [6-(3-Acetamidophenyl)-2-(4-acetamido-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-- methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Isopropanesulfonylamino-phenyl-sulfanyl)-6-phenyl-pyrimidin-4-yl]-(5- -methyl-2H-pyrazol-3-yl)-amine
- {2-[4-(2-Dimethylamino-acetylamino)-phenylsulfanyl]-6-phenyl-pyrimidin-4-y- l}-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-morpholin-4-yl-pyrimidin-4-yl]-(5-methyl-2H- -pyrazol-3-yl)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(2-methoxy-ethylamino)-pyrimidin-4-yl]-(5-m- ethyl-2H-pyrazol-3-yl)-amine
- [2-Benzylsulfanyl-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(5-methyl-2H-- pyrazol-3-yl)-amine
- [2-Benzylsulfanyl-6-morpholin-4-yl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-- yl)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(5- -methyl-2H-pyrazol-3-yl)-amine
- [2-(4-methoxy-benzylsulfanyl)-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(- 5-methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-6-tert-butyl-pyrimidin-4-yl]-(5-methyl-2H- -pyrazol-3-yl)-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[6-phenyl-2-(4-propionylamino-phenyl-sulfa- nyl)-pyrimidin-4-yl]-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(piperidin-1-yl)-pyrimidin-4-yl]-(5-methyl-- 2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-{2-[4-(morpholinesulfonyl)-benzylsulfanyl]-6-mo- rpholin-4-yl-pyrimidin-4-yl}-amine
- {6-(2-Methoxy-ethylamino)-2-[4-(morpholinesulfonyl)-benzylsulfanyl]-pyrimi- din-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- {6-(4-methylpiperazin-1-yl)-2-[4-(morpholinesulfonyl)-benzylsulfanyl]-pyri- midin-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- [6-Methoxymethyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-m- ethyl-2H-pyrazol-3-yl)-amine
- [2-(4-Methoxycarbonyl-phenyl-sulfanyl)-6-methoxymethyl-pyrimidin-4-yl]-(5-- methyl-2H-pyrazol-3-yl)-amine
- [2-(3,5-Dimethoxy-benzylsulfanyl)-6-morpholin-4-yl-pyrimidin-4-yl]-(5-meth- yl-2H-pyrazol-3-yl)-amine
- [2-(3,5-Dimethoxy-benzylsulfanyl)-6-pyrrolidin-4-yl-pyrimidin-4-yl]-(5-met- hyl-2H-pyrazol-3-yl)-amine
- 5-Methyl-2H-pyrazol-3-yl)-[6-morpholin-4-yl-2-(naphthalene-2-ylmethylsulf- anyl)-pyrimidin-4-yl]-amine
- {2-(4-Acetamido-phenyl-sulfanyl)-6-[4-(3-dimethylamino-propoxy)-phenyl]-py- rimidin-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamidophenylsulfanyl)-6-(morpholin-4-yl)-pyrimidin-4-yl]-(5-methy- l-2H-pyrazol-3-yl)-amine
- [6-Hydroxymethyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-m- ethyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-yl- )-amine
- [6-(1-Butoxycarbonyl)-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]- -(5-methyl-2H-pyrazol-3-yl)-amine
- [6-Methoxycarbonyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5- -methyl-2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-(6-phenyl-2-phenylamino-pyrimidin-4-yl)-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-(6-phenyl-2-phenylamino-pyrimidin-4-yl)-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[2-(3-methylphenylamino)-6-phenyl-pyrimidi- n-4-yl]-amine
- [2-(4-cyanomethylphenylamino)-6-phenyl-pyrimidin-4-yl]-(5-cyclopropyl-2H-p- yrazol-3-yl)-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[6-phenyl-2-(pyridin-3-ylmethylamino)-pyri- midin-4-yl]-amine
- [2-(3-Chlorophenyl)amino-6-(3-nitrophenyl)-pyrimidin-4-yl]-(5-methyl-2H-py- razol-3-yl)-amine
- [2-(3-Chlorophenyl)amino-6-(3,4,5-trimethoxyphenyl)-pyrimidin-4-yl]-(5-met- hyl-2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-[2-(4-sulfamoylphenylamino)-6-(3,4,5-trimethoxy- phenyl)-pyrimidin-4-yl]-amine
- [2-(4-Chlorophenyl)amino-6-methyl-pyrimidin-4-yl]-[5-(furan-2-yl)-2H-pyraz- ol-3-yl]-amine
- [2-(Benzimidazol-2-ylamino-)6-ethyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3- -yl)-amine
- [2-(4-Chlorophenyl)amino-6-methyl-pyrimidin-4-yl]-(5-phenyl-2H-pyrazol-3-y- l)-amine
- [2-(4-Chlorophenyl)amino-6-ethyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-yl- )-amine
- (5-tert-Butyl-2H-pyrazol-3-yl)-[2-(3-chlorophenyl)amino-6-(3-nitrophenyl)-- pyrimidin-4-yl]-amine
- [2-(3-Chlorophenyl)amino-6-(3-nitrophenyl)-pyrimidin-4-yl]-(5-phenyl-2H-py- razol-3-yl)-amine
- [5-(Furan-2-yl)-2H-pyrazol-3-yl]-(6-phenyl-2-phenylamino-pyrimidin-4-yl)-a- mine
- [2-(Benzimidazol-2-ylamino)-6-methyl-pyrimidin-4-yl]-(5-phenyl-2H-pyrazol-- 3-yl)-amine
- [2-(Benzimidazol-2-ylamino)-6-methyl-pyrimidin-4-yl]-[5-(Furan-2-yl)-2H-py- razol-3-yl]-amine
- [2-(4-Chlorophenylamino)-6-methyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-y- l)-amine
- [2-(4-Chlorophenyl)amino-5,6-dimethyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol- -3-yl)-amine
- (5,6-Dimethyl-2-phenylamino-pyrimidin-4-yl)-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Chlorophenyl)amino-6-methoxymethyl-pyrimidin-4-yl]-(5-methyl-2H-pyra- zol-3-yl)-amine
- [2-(Benzimidazol-2-ylamino)-6-methoxymethyl-pyrimidin-4-yl]-(5-methyl-2H-p- yrazol-3-yl)-amine
- (6-Methoxymethyl-2-phenylamino-pyrimidin-4-yl)-(5-methyl-2H-pyrazol-3-yl)-- amine
- (6-Methyl-2-phenylamino-pyrimidin-4-yl)-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(2-Chlorophenoxymethyl)-6-methyl-pyrimidin-4-yl]-(5-phenyl-2H-pyrazol-3- -yl)-amine
- [2-(2-Chlorophenoxymethyl)-6-methyl-pyrimidin-4-yl]-[5-(furan-2-yl)-2H-pyr- azol-3-yl]-amine
- (6-methyl-2-phenoxymethyl-pyrimidin-4-yl)-(5-phenyl-2H-pyrazol-3-yl)-amine
- [5-(Furan-2-yl)-2H-pyrazol-3-yl]-(6-methyl-2-phenoxymethyl-pyrimidin-4-yl)- -amine
- [5-(Furan-2-yl)-2H-pyrazol-3-yl]-(6-methyl-2-phenylsulfanylmethyl-pyrimidin-4-yl)-amine
- [6-Methyl-2-(4-methyl-phenylsulfanylmethyl)-pyrimidin-4-yl]-(5-phenyl-2H-p- yrazol-3-yl)-amine
- [5-(Furan-2-yl)-2H-pyrazol-3-yl]-[6-Methyl-2-(4-methyl-phenylsulfanylmethy- l)-pyrimidin-4-yl]-amine
- [2-(4-Fluoro-phenoxymethyl)-6-methyl-pyrimidin-4-yl]-(5-phenyl-2H-pyrazol-- 3-yl)-amine
- [2-(4-Fluoro-phenoxymethyl)-6-methyl-pyrimidin-4-yl]-[5-(furan-2-yl)-2H-py- razol-3-yl]-amine
- (6-Ethyl-2-phenylsulfanylmethyl-pyrimidin-4-yl)-(5-methyl-2H-pyrazol-3-yl)- -amine
- (6-Ethyl-2-phenoxymethyl-pyrimidin-4-yl)-(5-methyl-2H-pyrazol-3-yl)-amine
- [6-Ethyl-2-(4-fluorophenoxymethyl)-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-- yl)-amine
- [6-Ethyl-2-(1-methyl-1-phenyl-ethyl)-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-- 3-yl)-amine
- [2-(4-Chlororophenoxymethyl)-6-methyl-pyrimidin-4-yl]-(5-phenyl-2H-pyrazol- -3-yl)-amine
- [2-(4-Chlororophenoxymethyl)-6-methyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol- -3-yl)-amine
- [2-(4-Chlororophenoxymethyl)-6-methoxymethyl-pyrimidin-4-yl]-(5-methyl-2H-- pyrazol-3-yl)-amine
- [2-(4-Chlororophenoxymethyl)-6-methyl-pyrimidin-4-yl]-[5-(furan-2-yl)-2H-p- yrazol-3-yl]-amine
- (5-Methyl-2H-pyrazol-3-yl)-(2-phenylsulfanylmethyl-5,6,7,8-tetrahydro-quin- azolin-4-yl)-amine
- [2-(4-Methylphenylsulfanylmethyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidi- n-4-yl]-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(1-Methyl-1-phenyl-ethyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidin-4-y- l]-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(2,6-Dichlorobenzyl)-5,6,7,8-tetrahydro-quinazolin-4-yl]-(5-methyl-2H-p- yrazol-3-yl)-amine
- [7-Benzyl-2-(2,6-dichlorobenzyl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-- 4-yl]-(5-methyl-2H-pyrazol-3-yl)-amine
- [6-Benzyl-2-(4-chlorophenoxymethyl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimi- din-4-yl]-(5-methyl-2H-pyrazol-3-yl)-
- [2-(4-Chlorophenoxymethyl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-yl]- -(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(2,6-Dichlorobenzyl)-6-methyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-yl- )-amine
- [2-(2,6-Dichlorobenzyl)-5,6-dimethyl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-- 3-yl)-amine
Patent Number: US7008948 Applicant: Vertex Pharmaceuticals Incorporated Title: Fused pyrimidyl pyrazole compounds useful as protein kinase inhibitors Basic Structure
Derivatives of pyrimidine-pyrazole amine disclosed in the patent:
- [2-(2-Clorophenyl)-5,6-dimethylpyrimidin-4-yl]-(5-Methyl-2H-pyrazol-3-yl)-- amine
- [2-(2-Chloro-phenyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidin-4-yl]-(1H-i- ndazol-3-yl)-amine
- (5-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydr- o-pyrido[3,4-d]pyrimidin-4-yl]-
- [2-(2-Chloro-phenyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidin-4-yl]-(7-fl- uoro-1H-indazol-3-yl)-amine
- [2-(2-Chloro-phenyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidin-4-yl]-(5-fl- uoro-1H-indazol-3-yl)-amine
- [2-(2-Chloro-phenyl)-6,7,8,9-tetrahydro-5H-cycloheptapyrimidin-4-yl]-(5,7-- difluoro-1H-indazol-3-yl)-amine
- (7-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydr- oquinazolin-4-yl]-amine
- (5-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydr- oquinazolin-4-yl]-amine
- (5,7-Difluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetra- hydroquinazolin-4-yl]-amine
- (5-Trifluoromethyl-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-- tetrahydroquinazolin-4-yl]-amine
- (5,7-difluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-6,7,8,9-tetra- hydro-5H-cycloheptapyrimidin-4-yl]-amine
- (6-Benzyl-2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyr- imidin-4-yl)-(5-fluoro-1H-indazol-3-yl)-amine
- (1H-Indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-6,7,8,9-tetrahydro-5H-cycl- oheptapyrimidin-4-yl]-amine
- (7-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-6,7,8,9-tetrahydr- o-5H-cycloheptapyrimidin-4-yl]-amine
- (5-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-6,7,8,9-tetrahydr- o-5H-cycloheptapyrimidin-4-yl]-amine
- (5-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydr- o-pyrido[4,3-d]pyrimidin-4-yl]-amine
- (1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydroquinazol- in-4-yl]-amine
- (7-Benzyl-2-(2-trifluoromethyl-phenyl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-yl)-(5-fluoro-1H-indazol-3-yl)-amine
- (1H-Indazol-3-yl)-[6-methyl-2-(2-trifluoromethyl-phenyl)-pyrimidin-4-yl]-amine
- 1H-Indazol-3-yl)-[6-phenyl-2-(2-trifluoromethyl-phenyl)-pyrimidin-4-yl]-amine
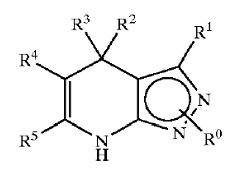
Patent Number: US6977262 Assignee: Mitsubishi Pharma Corporation Title: Dihydropyrazolopyridine compounds and pharmaceutical use thereof Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in the patent:
- Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- Ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- Ethyl 4,7-dihydro-6-methyl-4-(2-trifluoromethylphenyl)-2H-pyrazolo[3,4-b]pyridin e-5-carboxylate
- Methyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- t-Butyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- Isopropyl 4-(2-fluorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- Benzyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
- 4-(2-Chlorophenyl)-5-dimethylaminocarbonyl-4,7-dihydro-6-methyl-2H-pyrazolo [3,4-b]pyridine
- 4-(2-Chlorophenyl)-5-hydrazinocarbonyl-4,7-dihydro-6-methyl-2H-pyrazolo[3,4 -b]pyridine
- 4-(2-Fluorophenyl)-4,7-dihydro-6-methyl-5-isopropylthiocarbonyl-2H-pyrazolo [3,4-b]pyridine
- 4,7-Dihydro-6-methyl-5-nitro-4-(2-trifluoromethylphenyl)-2H-pyrazolo[3,4-b] pyridine
- Ethyl 4,7-dihydro-4-phenyl-6-trifluoromethyl-2H-pyrazolo[3,4-b]pyridine-5-carbox ylate
- Ethyl 4-(2-fluorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-pyrazolo[3,4-b]pyridin e-5-carboxylate
- Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-pyrazolo[3,4-b]pyridin e-5-carboxylate maleate
- Ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-trifluoromethyl-2H-pyrazolo[3,4-b]pyridi ne-5-carboxylate
- Ethyl 4,7-dihydro-6-trifluoromethyl-4-(2-trifluoromethylphenyl)-2H-pyrazolo[3,4- b]pyridine-5-carboxylate
- Ethyl 4-(3-chlorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-pyrazolo[3,4-b]pyridin e-5-carboxylate
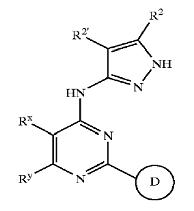
Patent Number: US6664247 Assignee: Vertex Pharmaceuticals Incorporated Title: Pyrazole compounds useful as protein kinase inhibitors Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in the patent:
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[2-(naphtalen-2-ylsulfanyl)-6-phenylpyrimid in-4-yl]-amine
- 5-Cyclopropyl-2H-pyrazol-3-yl)-[2-(3-methoxycarbonyl-phenylylsulfanyl)-6-p henylpyrimidin-4-yl]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[2-(naphthalen-2-ylsulfanyl)-pyrimidin-4-yl ]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[5,6-dimethyl-2-(naphthalen-2-ylsulfanyl)-p yrimidin-4-yl]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[5-methyl-2-(naphthalen-2-ylsulfanyl)-pyrim idin-4-yl]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[6-methyl-2-(naphthalen-2-ylsulfanyl)-pyrim idin-4-yl]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[6-(morpholin-4-yl)-2-(naphthalen-2-ylsulfa nyl)-pyrimidin-4-yl]-amine
- (5-Cyclopropyl-2H-pyrazol-3-yl)-[6-(1-methylpiperazin-4-yl)-2-(naphthalen-2 -ylsulfanyl)-pyrimidin-4-yl]-amine
- [6-(2,6-Dimethylphenyl)-2-(naphthalen-2-ylsulfanyl)-pyrimidin-4-yl]-(5-meth yl-2H-pyrazol-3-yl)-amine
- [6-(2-Methylphenyl)-2-(naphthalen-2-ylsulfanyl)-pyrimidin-4-yl]-(5-methyl-2 H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenylsulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-methyl-2H-pyraz ol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-[2-(naphthalen-2-ylsulfanyl)-6-phenyl-pyrimidin- 4-yl]-amine
- [2-(4-Isobutyrylylamino-phenylsulfanyl)-6-phenylpyrimidin-4-yl]-(5-methyl-2 H-pyrazol-3-yl)-amine
- [6-(4-Methylpiperazin-1-yl)-2-methylsulfanyl-pyrimidin-4-yl]-(5-methyl-2H-p yrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-[6-phenyl-2-(4-propionylamino-phenylsulfanyl)-py rimidin-4-yl]-amine
- [2-(4-Cyclopropanecarbonylamino-phenylsulfanyl)-6-phenylpyrimidin-4-yl]-(5- methyl-2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-{6-phenyl-2-[4-(propane-1-sulfonylamino)-phenyls ulfanyl]-pyrimidin-4-yl}-amine
- [2-(4-Ethanesulfonylamino-phenylsulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-methy l-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamidophenyl-sulfanyl)-6-(2-methylphenyl)-pyrimidin-4-yl]-(5-methy l-2H-pyrazol-3-yl)-amine
- [2-(4-Isobutanecarbonylamino-phenyl-sulfanyl)-6-phenyl-pyrimidin-4-yl]-(5-m ethyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-5-methyl-6-phenyl-pyrimidin-4-yl]-(5-methy l-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-6-(4-methoxyphenyl)-pyrimidin-4-yl]-(5-met hyl-2H-pyrazol-3-yl)-amine
- [6-(3-Acetamidophenyl)-2-(4-acetamido-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-m ethyl-2H-pyrazol-3-yl)-amine
- [2-(4-Isopropanesulfonylamino-phenyl-sulfanyl)-6-phenyl-pyrimidin-4-yl]-(5- methyl-2H-pyrazol-3-yl)-amine
- (2-[4-(2-Dimethylamino-acetylamino)-phenylsulfanyl]-6-phenyl-pyrimidin-4-yl }-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-morpholin-4-yl-pyrimidin-4-yl]-(5-methyl-2H- pyrazol-3-yl)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(2-methoxy-ethylamino)-pyrimidin-4-yl]-(5-me thyl-2H-pyrazol-3-yl)-amine
- [2-Benzylsulfanyl-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(5-methyl-2H-p yrazol-3-yl)-amine
- [2-Benzylsulfanyl-6-morpholin-4-yl-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-y l)-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(5- methyl-2H-pyrazol-3-yl)-amine
- [2-(4-methoxy-benzylsulfanyl)-6-(4-methylpiperazin-1-yl)-pyrimidin-4-yl]-(5 -methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-6-tert-butyl-pyrimidin-4-yl]-(5-methyl-2H- pyrazol-3-yl)-amine
- 5-Cyclopropyl-2H-pyrazol-3-yl)-[6-phenyl-2-(4-propionylamino-phenyl-sulfan yl)-pyrimidin-4-yl]-amine
- [2-(3-Chloro-benzylsulfanyl)-6-(piperidin-1-yl)-pyrimidin-4-yl]-(5-methyl-2 H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-{2-[4-(morpholinesulfonyl)-benzylsulfanyl]-6-mor pholin-4-yl-pyrimidin-4-yl}-amine
- {6-(2-Methoxy-ethylamino)-2-[4-(morpholinesulfonyl)-benzylsulfanyl]-pyrimid in-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- {6-(4-methylpiperazin-1-yl)-2-[4-(morpholinesulfonyl)-benzylsulfanyl]-pyrim idin-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- [6-Methoxymethyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-me thyl-2H-pyrazol-3-yl)-amine
- [2-(4-Methoxycarbonyl-phenyl-sulfanyl)-6-methoxymethyl-pyrimidin-4-yl]-(5-m ethyl-2H-pyrazol-3-yl)-amine
- [2-(3,5-Dimethoxy-benzylsulfanyl)-6-morpholin-4-yl-pyrimidin-4-yl]-(5-methy l-2H-pyrazol-3-yl)-amine
- [2-(3,5-Dimethoxy-benzylsulfanyl)-6-pyrrolidin-4-yl-pyrimidin-4-yl]-(5-meth yl-2H-pyrazol-3-yl)-amine
- (5-Methyl-2H-pyrazol-3-yl)-[6-morpholin-4-yl-2-(naphthalene-2-yl-methylsulf anyl)-pyrimidin-4-yl]-amine
- {2-(4-Acetamido-phenyl-sulfanyl)-6-[4-(3-dimethylamino-propoxy)phenyl]-pyri midin-4-yl}-(5-methyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamidophenylsulfanyl)-6-(morpholin-4-yl)-pyrimidin-4-yl]-(5-methyl -2H-pyrazol-3-yl)-amine
- [6-Hydroxymethyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-me thyl-2H-pyrazol-3-yl)-amine
- [2-(4-Acetamido-phenyl-sulfanyl)-pyrimidin-4-yl]-(5-methyl-2H-pyrazol-3-yl) -amine
- [6-(1-Butoxycarbonyl)-2-(4-propionylamino-phenyl-sulfanyl)pyrimidin-4-yl]-( 5-methyl-2H-pyrazol-3-yl)-amine
- [6-Methoxycarbonyl-2-(4-propionylamino-phenyl-sulfanyl)-pyrimidin-4-yl]-(5- methyl-2H-pyrazol-3-yl)-amine.
Patent Number: US2004224944 Assignee: VERTEX PHARMACEUTICALS INC Title: Pyrazole compounds useful as protein kinase inhibitors Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in the patent:
- [2-(2-Chloro-phenyl)-6,7-dihydro-5H-cyclopentapyrimidin-4-yl]-(5,7-difluoro-1H-indazol-3-yl)-amine
- (1H-Indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8,9,10-hexahydro-cyclooctapyrimidin-4-yl]-amine
- (7-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8,9,10-hexahydro-cyclooctapyrimidin-4-yl]-amine
- (5,7-Difluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-5,6,7,8,9,10-hexahydro-cyclooctapyrimidin-4-yl]-amine
- [6-Cyclohexyl-2-(2-trifluoromethyl-phenyl)-pyrimidin-4-yl]-(1H-indazol-3-yl)-amine
- [6-(2-Fluoro-phenyl)-2-(2-trifluoromethyl-phenyl)-pyrimidin-4-yl]-(1H-indazol-3-yl)-amine
- (6-Fluoro-1H-indazol-3-yl)-[2-(2-trifluoromethyl-phenyl)-quinazolin-4-yl]-amine
- 3-[2-(2-Trifluoromethyl-phenyl)-quinazolin-4-ylamino]-1H-indazole-5-carboxylic acid methyl ster
- (5-Methyl-2H-pyrazol-3-yl)-[2-(2-naphthyl-1-yl)-quinazolin-4-yl]-
- [2-(2-Chloro-phenyl)-pyrido[2,3-d]pyrimidin-4-yl]-(7-fluoro-1H-indazol-3-yl)-amine
- [2-(2-Chloro-phenyl)-pyrido[2,3-d]pyrimidin-4-yl]-(5-fluoro-1H-indazol-3-yl)-amine
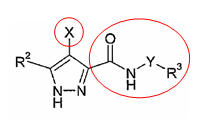
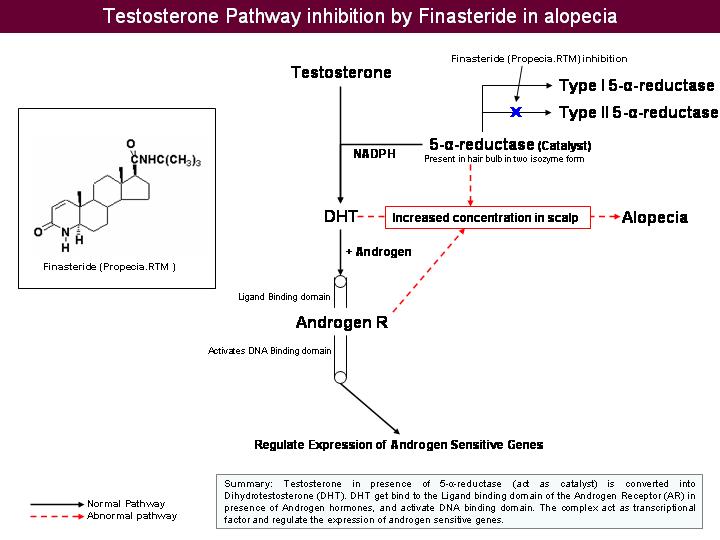
Inhibition of 5- - reductase by Finasteride
Other derivates used by different assigness for the treatment of alopecia
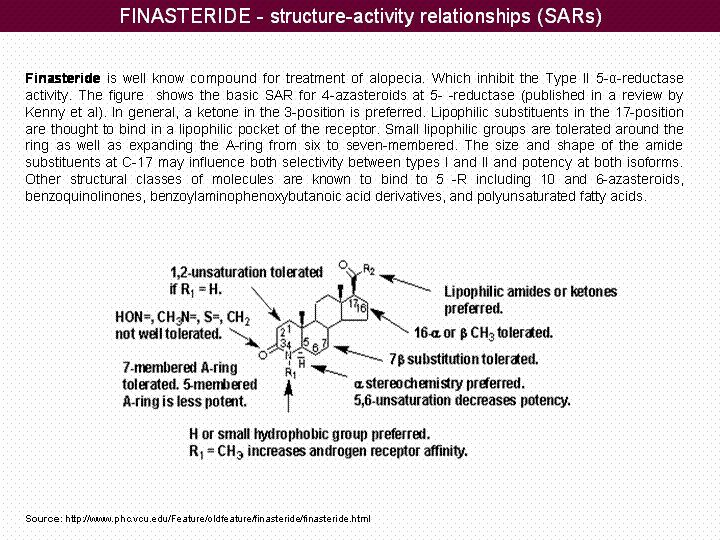
Structure-Activity Relationships (SARs) of Finasteride
Questions Dolcera Answers
What’s hot?
- What compositions/ approaches are the most promising?
- What can I license?
- Can you map blockbuster products to their patents?
Can you save me some time?
- What combinations/ compounds have already been tried?
- Is any empirical data available?
- Can you tell me the side effects?
Where should I focus my R&D investment?
- What are the most promising approaches?
- Where’s the ‘white space’ for me to play in?
Any hints for research?
- Are there any combinations I could develop?
What should I do in this geography?
- What are my competitors up to in this geography?
- What are my strengths/ weaknesses here?
What’s my competition up to?
- What’s my top competitor investing in?
- Are there any loopholes in their patents?
- When are their patents expiring?
- Will a competitor emerge from nowhere and surprise me?
- What are the crowded areas?
How do I play defense?
- What should my blocking/reactive strategies be?
New Combinations based on IP study?
Yes, new Combinations can be made with natural products based on IP study
- Walnut extract containing
 inhibitor (as anti-androgen) and Flavnones (for vasodilation).
inhibitor (as anti-androgen) and Flavnones (for vasodilation). - Sophora Flavnones (for vasodilation) in combination with Saw Palmetto berry (as anti-androgen).
Note: The above combinations are based on limited study and are only possible examples
IP studies provides
Technology trends
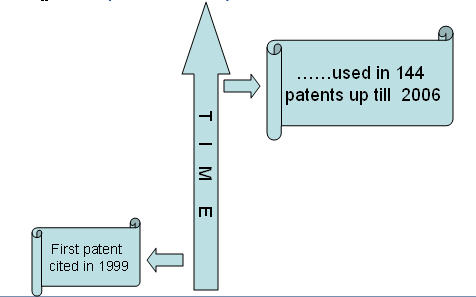
- Use of saw palmetto berry for treating alopecia was first patented in 1996.
- Since then, 144 patents (including family patents) have been filed till 2006.
- Most patents use saw palmetto berry in combination with other products.
New opportunities
Yes, the IP studies provide new opportunities in the following area.
- Sophora Flavescens contain flavnoids.
- Natural extract Sophora Flavescens cited in LG patent of 2001.
- Research shows fewer than 7 patents based on Sophora Flavescens for hair loss or alopecia.
Conclusions
- Hair loss medication is a very active area of research and intellectual property development.
- One of the most promising areas of development is the area of Anti-androgens.
- The top companies are Merck, L’Oreal and Smithkline.