Vaccines Market in Western Countries
Contents
- 1 Summary
- 2 Countries Considered for Market Assessment
- 3 Market Size of Vaccines Market
- 4 Current State of Exports of Vaccines Shipments from India
- 5 Vaccine market in USA
- 5.1 Pediatric Vaccine
- 5.2 Adult Vaccine
- 5.3 Influenza Vaccine
- 6 Vaccines Market in United States
- 7 GlaxoSmithKline profile
- 8 Assessment of Various Entry methods for Serum India
Summary
Countries Considered for Market Assessment
The following markets were considered for assessment :
- The United States of America
- Europe
For the purpose of this study, Europe was defined as comprising of the following countries: Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia & Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Macedonia, Malta, Moldova, Monaco, The Netherlands, Norway, Poland, Portugal, Romania, Russia, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Ukraine, and United Kingdom.
After initial study, the focus was then confined to the US market.
Market Size of Vaccines Market
Key Highlights
- Global sales of human and animal vaccines reached approximately $25.2 billion in 2009. The market is expected to rise at a compound annual growth rate (CAGR) of 17.9% to reach $64.2 billion by 2015.
- The global market for human vaccines in 2008 was $18.7 billion and, with a continuing increase in the global market, reached $21.2 billion in 2009. This market is expected to rise at a compound annual growth rate (CAGR) of 19.6% to reach $58.6 billion by the end of 2015.
- The animal vaccines market grew from approximately $3.8 billion in 2008 to about $4.0 billion in 2009, an increase of about 5.9%. This market is expected to increase at a 5.7% compound annual growth rate (CAGR) between 2010 and 2015 to reach $5.6 billion in 2015.
Source: bcc-research
Market Size in terms of Number of Vaccines Required by Country
Infant Vaccine Market
Pediatric vaccines are given to all children depending upon the vaccination schedule of the country. Disease incidence and prevalence rates have little bearing on the infant vaccine market size.
| S. No. | Name | Population | Birthrate | No. Of Infants Every Year |
| 1 | Albania | 3,155,271 | 15.29 | 48,244 |
| 2 | Andorra | 851,68 | 10.35 | 881 |
| 3 | Armenia | 3,082,951 | 12.65 | 38,999 |
| 4 | Austria | 8,364,095 | 8.65 | 72,349 |
| 5 | Azerbaijan | 8,781,100 | 17.62 | 154,723 |
| 6 | Belarus | 9,663,000 | 9.71 | 93,828 |
| 7 | Belgium | 10,788,760 | 10.15 | 109,506 |
| 8 | Bosnia & Herzegovina | 3,766,579 | 8.85 | 33,334 |
| 9 | Bulgaria | 7,585,131 | 9.51 | 72,135 |
| 10 | Croatia | 4,432,001 | 9.64 | 42,724 |
| 11 | Cyprus | 87,1036 | 12.57 | 10,949 |
| 12 | Czech Republic | 10,489,970 | 8.83 | 92,626 |
| 13 | Denmark | 5,529,270 | 10.54 | 58,279 |
| 14 | Estonia | 1,340,345 | 10.37 | 13,899 |
| 15 | Finland | 5,338,395 | 10.38 | 55,413 |
| 16 | France | 62,616,488 | 12.57 | 787,089 |
| 17 | Georgia | 8,186,453 | 10.66 | 87,268 |
| 18 | Germany | 81,879,976 | 8.18 | 669,778 |
| 19 | Greece | 11,283,293 | 9.45 | 106,627 |
| 20 | Hungary | 10,022,302 | 9.51 | 95,312 |
| 21 | Iceland | 319,062 | 13.43 | 4,285 |
| 22 | Ireland | 4,450,446 | 14.23 | 63,330 |
| 23 | Italy | 60,221,211 | 8.18 | 492,610 |
| 25 | Latvia | 2,255,128 | 9.78 | 22,055 |
| 26 | Liechtenstein | 35,911 | 9.75 | 350 |
| 27 | Lithuania | 3,339,550 | 9.11 | 30,423 |
| 28 | Luxembourg | 497,854 | 11.73 | 5,840 |
| 29 | Macedonia | 2,042,484 | 11.97 | 24,449 |
| 30 | Malta | 414,971 | 10.36 | 4,299 |
| 31 | Moldova | 3,603,506 | 11.12 | 40,071 |
| 32 | Monaco | 32,812 | 9.1 | 299 |
| 34 | The Netherlands | 16,531,294 | 10.4 | 171,925 |
| 35 | Norway | 4,827,038 | 10.99 | 53,049 |
| 36 | Poland | 38,149,886 | 10.04 | 383,025 |
| 37 | Portugal | 10,632,069 | 10.29 | 109,404 |
| 38 | Romania | 21,482,395 | 10.53 | 226,210 |
| 39 | Russia | 141,850,000 | 11.1 | 1,574,535 |
| 40 | San Marino | 31,451 | 9.63 | 303 |
| 41 | Serbia | 7,319,712 | 9.19 | 67,268 |
| 42 | Slovakia | 5,418,156 | 10.6 | 57,432 |
| 43 | Slovenia | 2,043,241 | 8.97 | 18,328 |
| 44 | Spain | 45,957,671 | 9.72 | 446,709 |
| 45 | Sweden | 9,000,000 | 10.13 | 91,170 |
| 46 | Switzerland | 7,731,167 | 9.59 | 74,142 |
| 47 | Turkey | 74,815,703 | 18.66 | 1,396,061 |
| 48 | Ukraine | 46,008,406 | 9.6 | 441,681 |
| 49 | United Kingdom | 61,838,154 | 10.65 | 658,576 |
| 50 | United States of America | 307,006,550 | 13.82 | 4,242,831 |
| 51 | Canada | 33,739,900 | 10.28 | 346,846 |
Based on number of mandatory vaccine shots in each of European country, following section lists 10 biggest markets in each category of vaccine
| Top 10 Markets for Diphtheria in Europe |
| France |
| Germany |
| United Kingdom |
| Spain |
| Italy |
| Poland |
| Romania |
| The Netherlands |
| Belgium |
| Portugal |
| Top 10 Markets for IPV in Europe |
| France |
| Germany |
| United Kingdom |
| Italy |
| Spain |
| Poland |
| The Netherlands |
| Belgium |
| Hungary |
| Czech Republic |
| Top 10 Markets for MMR in Europe |
| France |
| Germany |
| United Kingdom |
| Poland |
| Italy |
| Spain |
| Romania |
| Belgium |
| The Netherlands |
| Austria |
| Top 10 Markets for Influenza in Europe |
| France |
| Germany |
| United Kingdom |
| Spain |
| Poland |
| Italy |
| Romania |
| The Netherlands |
| Belgium |
| Greece |
| Top 10 Markets for Hep B in Europe |
| France |
| Germany |
| Spain |
| Poland |
| Italy |
| Romania |
| Belgium |
| Portugal |
| Czech Republic |
| Austria |
| Top 10 Markets for BCG in Europe |
| France |
| Germany |
| United Kingdom |
| Poland |
| Romania |
| Portugal |
| Hungary |
| Czech Republic |
| Slovakia |
| Finland |
Market Size in terms of Value of the Market
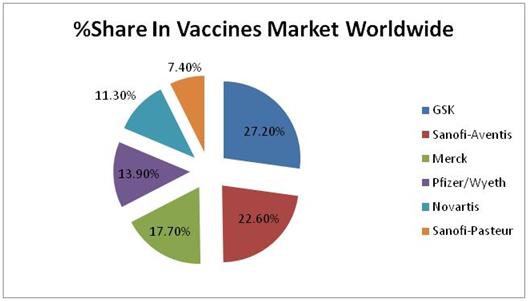
In 2009 Market Size of Vaccines Market was about USD 21 Billion
Relative Market Shares of top 6 companies are shown below :
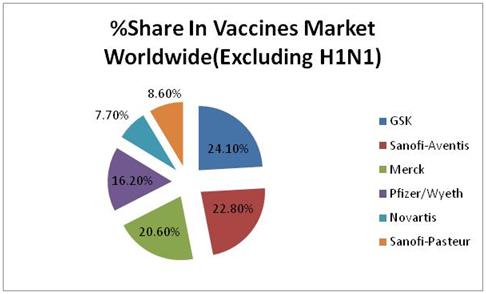
Excluding H1N1 relative market share of top 6 companies is as follows :
Current State of Exports of Vaccines Shipments from India
Europe
| HS Code | Description | 2005-2006 | 2006-2007 | 2007-2008 | 2008-2009 | 2009-2010 | 2010-2011(Apr-Sep) |
| 30022011 | Vaccines for Cholera and Typhoid | 0 | 0.4 | 4.41 | 4.81 | 0.11 | 0 |
| 30022012 | Vaccines for Hepatitis | 7.4 | 7.18 | 11.73 | 0.25 | 0.08 | 0 |
| 30022013 | Vaccines for Tetanus | 0.13 | 1.35 | 0.43 | 0.25 | 0.05 | 0 |
| 30022014 | Vaccines for Polio | 0 | 0 | 0 | 0 | 0 | 0 |
| 30022015 | Vaccines for Tuberculins (B.C.G.) | 0 | 0 | 0.89 | 0.09 | 0 | 0.03 |
| 30022016 | Anti Rabies Vaccines | 0.02 | 0.01 | 0.2 | 0 | 0 | 0 |
| 30022018 | Vaccines for Whooping Cough (Pertusis) | 0 | 0 | 0 | 0 | 0 | 0 |
| 30022019 | Other Single Vaccine | 0.16 | 1.78 | 0.1 | 1 | 1.12 | 2.18 |
| 30022021 | Mixed Vaccines for DPT - Triple Anti Gen | 0.67 | 0.96 | 0 | 0 | 0.08 | 0 |
| 30022022 | Mixed Vaccines for Diphtheria and Tetanus | 0.11 | 1.06 | 0 | 0.46 | 0 | 0.04 |
| 30022023 | Mixed Vaccines for M.M.R. | 3.7 | 2.36 | 0 | 0.83 | 0.39 | 1.03 |
| 30022024 | Mixed Vaccines for T.A.B. or T.A.B.C. | 0 | 0 | 0 | 0 | 0 | 0 |
| 30022029 | Other Mixed Vaccines | 0.31 | 0.16 | 0.18 | 0.04 | 0.13 | 0.95 |
| 300220 | All Vaccines for Human Medicine | 12.52 | 15.26 | 17.95 | 7.74 | 1.97 | 4.23 |
USA
| HS Code | Description | 2005-2006 | 2006-2007 | 2007-2008 | 2008-2009 | 2009-2010 | 2010-2011(Apr-Sep) |
| 30022011 | Vaccines for Cholera and Typhoid | 0 | 0 | 0.7 | 0.02 | 0 | 0 |
| 30022012 | Vaccines for Hepatitis | 0.02 | 0.02 | 0.09 | 0 | 0.15 | 0 |
| 30022013 | Vaccines for Tetanus | 0 | 0.13 | 0.04 | 0 | 0 | 0 |
| 30022014 | Vaccines for Polio | 0 | 0.05 | 0 | 0 | 0 | 0 |
| 30022015 | Vaccines for Tuberculins (B.C.G.) | 0 | 0 | 0 | 0 | 0 | 0 |
| 30022016 | Anti Rabies Vaccines | 0 | 0.06 | 0.06 | 0 | 0 | 0 |
| 30022018 | Vaccines for Whooping Cough (Pertusis) | 0 | 0 | 0 | 0.27 | 0 | 0.01 |
| 30022019 | Other Single Vaccine | 0.33 | 0.05 | 1.73 | 0.05 | 0.05 | 0 |
| 30022021 | Mixed Vaccines for DPT - Triple Anti Gen | 0.04 | 0.09 | 0 | 0 | 0 | 0 |
| 30022022 | Mixed Vaccines for Diphtheria and Tetanus | 0 | 0.09 | 0 | 0 | 0 | 0 |
| 30022023 | Mixed Vaccines for M.M.R. | 0.14 | 0.01 | 0 | 0 | 0 | 0 |
| 30022024 | Mixed Vaccines for T.A.B. or T.A.B.C. | 0 | 0 | 0 | 0 | 0 | 0 |
| 30022029 | Other Mixed Vaccines | 0.97 | 0.16 | 0.06 | 0.22 | 0.01 | 0 |
| 300220 | All Vaccines for Human Medicine | 1.49 | 0.66 | 2.66 | 0.54 | 0.21 | 0.01 |
All figures in million USD
Source: [Department of Commerce, Government of India]
Vaccine market in USA
Pediatric Vaccine
Diphtheria, Tetanus, Pertussis
The combination vaccine, DTaP, which protects against diphtheria-tetanus-acellular pertussis has been on the market since early 1991. The original pertussis vaccine (DTP) included parts of the entire bacterial cell that causes pertussis. Research has shown that only certain parts of the bacterial cell—not the whole bacterial cell—are required to produce immunity. DTaP is called an acellular vaccine since it contains only those proteins that are important for producing immunity and not the entire bacterial cells. DTaP causes fewer side effects than the entire cell vaccine and is recommended for all children because of the reduced risk of adverse reactions when compared with DTP. Whole-cell DTP is no longer recommended for use in the United States.
DTaP products which are included and currently available are Infanrix, Daptacel and Tripedia. Any of these products can be used for all doses in the vaccination series. It is expected that the usage of whole-cell pertussis products (DTP) will decrease in favor of the improved DTaP products over the coming years. However, DTP products continue to be used in others parts of the world.
These include :
- Alditerpera (Sevac, Czechoslovakia);
- D.S.D.P.T. (Dong Shin Pharm, Korea);
- Sii Triple Antigen (Serum Institute, India);
- Triple (Cuba);
- Trivacuna Leti (Laboratory Leti, Spain).
In December 2002, Pediarix (GlaxoSmithKline) was approved by the FDA, becoming the first U.S.-licensed vaccine to offer protection against five diseases simultaneously and thereby reducing the total number of shots infants receive by up to six. This was an important improvement, since children often receive as many as 24 shots of various vaccines in the first two years itself. In fact, a Harris Interactive survey of 1,053 mothers found that almost 90% preferred a combination vaccine to individual immunizations, as combination vaccines can decrease the number of doctor visits and make the inoculation process more tolerable for children. Pediarix prevents diphtheria, tetanus toxoids, acellular pertussis adsorbed, hepatitis B (Recombinant) and inactivated poliovirus, and represents a next-generation product to GlaxoSmithKline’s Infanrix, which covers just three of these diseases (diphtheria and tetanus toxoids and acellular pertussis). Pediarix is approved as a 3-dose primary series, at 2,4 and 6 months of ages. It is licensed for children with age gropus between 6 weeks to 6 years of age, and should not be given to infants less than 6 weeks old or to anyone 7 years of age or older.
In 2005, GlaxoSmithKline launched Boostrix in the U.S., a vaccine that adds protection against pertussis to the routine tetanus/diptheria booster administered to teenagers. In June 2005, Sanofi Pasteur’s Adacel was approved for use in the United States by persons aged 11 to 64 years. Adacel is a tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine.
In June 2008, the FDA approved Sanofi Pasteur’s Pentacel which contains pertussis, diphtheria, tetanus, polio and Haemophilus influenzae type B. As of mid 2010, it remained the first and only vaccine to prevent against DTaP-IPV/Hib. The product, which is indicated for infants and children falling in the age group between 6 weeks and 4 years (prior to fifth birthday), is also available in Europe, Canada and other regions.
Also in mid 2008, the FDA approved GlaxoSmithKline’s Kimrix, the first combination vaccine to offer protection against diphtheria, tetanus, pertussis and polio diseases in one shot. The new vaccine contains the same DtaP and IPV components used in Infanrix and Pediarix.
In early 2009, India-based Bharat Biotech launched COMVAC5, a single-shot pentavalent combination vaccine which contains the first indigenously developed and manufactured haemophilus influenza type-b (Hib) vaccine in India and the only hepatitis B vaccine in the world to be manufactured without the use of cesium chloride. COMVAC5 protects against diphtheria, pertussis, tetanus, hepatitis B and Hib.
Diphtheria
Named in 1826 by French physician Pierre Bretonneau, diphtheria is a highly contagious bacterial infection caused by Corynebacterium diphtheriae, transmitted through close physical and respiratory contact. It can cause infection of the nasopharynx, causing a leathery, sheet like membrane to grow on the tonsils, throat, and in the nose which subsequently causes difficulty in breathing and even death. Prior to widespread immunization, diphtheria was a cyclical epidemic disease that caused frequent large scale outbreaks. For example, an outbreak in New England between 1735 and 1740 was reported to have killed 80% of the children under ten years of age in some areas. With increasing immunization in all countries, epidemics have given way to sporadic cases and intermittent outbreaks of low intensity.
Pertussis
Also highly contagious, pertussis (whooping cough) is a disease caused by Bordetella pertussis. Worldwide, this bacterial agent causes 20 to 40 million cases of pertussis and an estimated 200,000 to 300,000 fatalities each year. In the U.S., cases of pertusis are rising, with a tenfold increase in reported adolescent cases in the last decade, such that adolescents now account for almost 40% of all pertusis cases. Pertusis is believed to be significantly under-reported due to failure of both diagnosis and reporting of the condition. Although pertussis may occur at any age, most cases of serious disease and fatality are observed in early infancy. As a result, the American Academy of Family Physicians (AAFP) launched a campaign for pertusis awareness and vaccination in August 2009 that designed to help reduce the 600,000 cases that now occur each year in the U.S.
Vaccines are the most effective approach to pertussis control. For several decades inactivated whole cell vaccines have been part of national childhood vaccination programs, dramatically reducing the considerable public health impact of pertussis. These vaccines are currently being produced in over 40 countries, including many
Tetanus
Tetanus is the only vaccine-preventable disease that is not communicable; rather it is acquired through environmental exposure to the spores of Clostridium tetani. The disease is caused by the action of a potent neurotoxin produced during the anaerobic growth of the bacterium in necrosed tissues such as dirty wounds. Clinical symptoms of tetanus are muscle spasms, initially muscles of mastication causing trismus or ‘lockjaw’, which results in a characteristic facial expression – ‘risus sardonicus’. Trismus can be followed by spasms of other muscles, such as sustained spasm of back muscles - ‘opisthotonus’. Finally, mild external stimuli may trigger generalized tonic tetanic seizure-like activity, which contributes to the serious complications of tetanus (dysphagia, aspiration pneumonia) leading to death unless intense supportive treatment is immediately initiated.
Neonatal tetanus is the most common form of tetanus in developing countries. The disease is caused by contamination of the umbilical stump with spores following childbirth through cutting the cord with a non-sterile instrument or by application of animal dung to the cut cord. Symptoms begin 3 to 14 days after birth following a period of normal feeding. The infant suddenly fails to suck properly and becomes irritable; convulsions occur with increasing frequency and intensity.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | Tripedia® | DTaP | $13.25 | $23.68 |
| Sanofi Pasteur | Daptacel® | DTaP | $14.51 | $24.40 |
| GlaxoSmithKline | Infanrix® | DTaP | $14.85 | $20.96 |
| GlaxoSmithKline | Kinrix® | DTaP-IPV | $34.25 | $48.00 |
| GlaxoSmithKline | Pediarix® | DTaP-Hep B-IPV | $51.15 | $70.72 |
| Sanofi Pasteur | Pentacel® | DTaP-IP-HI | $52.55 | $77.48 |
| Sanofi Pasteur | Decavac® | Tetanus & Diphtheria Toxoids | $16.50 | $20.39 |
| MassBiologics | MassBiologics | Tetanus & Diphtheria Toxoids | $15.00 | Not sold to private sector |
| GlaxoSmithKline | Boostrix® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $29.59 | $37.55 |
| Sanofi Pasteur | Adacel® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $29.59 | $38.83 |
The end date for these contracts is March 31, 2012.
Haemophilus Influenzae Type B
Haemophilus influenzae type b (Hib) continues to be a leading cause of childhood meningitis and a major cause of bacterial pneumonia in children. Although little population based incidence data are available for most of Asia and the former Soviet Union, Hib is estimated to cause atleast 3 million cases of serious disease and over 380,000 deaths annually, worldwide. The most important manifestations of Hib disease, namely pneumonia and meningitis, are seen primarily in children under five years of age.
Currently, several different Hib vaccines, all conjugate vaccines, are available. These vaccines have shown protective efficacy in early infancy. Hib vaccines are now used as part of routine childhood vaccination programs in more than 20 countries including Australia, Canada, New Zealand, the United States, and many countries of Western Europe. They have proven to be highly efficacious and virtually free from serious side-effects.
The major manufacturers of Hib vaccines include :
- GlaxoSmithKline (Hiberix);
- Merck (PedvaxHIB);
- Novartis (Quattvaxem, Vaxem-HIB);
- Sanofi Pasteur (Act-HIB);
Also available is Merck’s Comvax, which is a combination of the company’s PedvaxHIB vaccine and its Recombivax HB. Wyeth (which is now part of Pfizer) had previously offered HibTITER, but discontinued this product in mid 2007, is available from Bharat Biotech in India and Korea on a limited basis.
This, along with Merck’s temporary discontinuation of PedvaxHIB from 2007 to 2009 on production equipment contamination, contributed to a U.S. vaccine shortage, leading the Centers for Disease Control (CDC) to recommend temporary deferral of the booster dose for healthy children who are not at increased risk for Hib disease. In mid 2009, the CDC reinstated the routine Hib booster dose due to an increase in supply. However, supply was not sufficient to support a mass catch-up effort for the millions of children who did not receive the booster dose during the shortage. The FDA therefore issued an accelerated approval in August 2009 for GlaxoSmithKline’s Hiberix as a booster dose in children 15 months through four years of age.
In May 2009, Novartis entered into an agreement with Takeda Pharmaceutical Company to distribute Vaxem-HIB in Japan. Through this agreement, Novartis will manufacture the vaccine and supply it to Takeda, which will obtain exclusive rights to license, market and distribute the vaccine in Japan. Takeda will become the exclusive partner for the product in Japan, and will be responsible for conducting clinical trials and submitting a New Drug Application (NDA). Following approval, Takeda will label the product and sell the vaccine under the Novartis brand name. The deal represents the entrance of Novartis into the Japanese vaccine market and is likely to be followed by other vaccine products.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | Adacel® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $26.25 | $38.83 |
| GlaxoSmithKline | Boostrix® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $26.25 | $37.55 |
| Sanofi Pasteur | Decavac® | Tetanus and Diphtheria Toxoids | $13.82 | $20.39 |
The end date for these contracts is March 31, 2012.
Hepatitis A
Hepatitis A is an acute disease of the liver caused by hepatitis A virus (HAV). It is transmitted from person to person, primarily by the fecal-oral route. Young children who catch HAV often have a milder form of the disease, usually lasting from 1 - 3 weeks, while adults tend to experience a much more severe form of the disease. They are often confined to bed for about 4 weeks and must stop work for 1 – 3 months or more. Many adults take up to a year to recover entirely and typically experience low immunity in the following months. This often represents a substantial medical and economic burden.
The incidence of hepatitis A is closely related to socioeconomic development, and studies show that prevalence of anti-HAV antibodies in the general population varies from 15% to close to 100% in different parts of the world. An estimated 1.5 million clinical cases of HAV occur each year. In areas of low endemicity, hepatitis A usually occurs as single cases among persons in high-risk groups or as outbreaks involving a small number of persons. In areas of high endemicity, most persons are infected with HAV without symptoms during childhood. In countries of low and intermediate disease endemicity, adult disease is seen more often.
Leading vaccines against HAV include Havrix (GlaxoSmithKline), Vaqta (Merck), and Avaxim (Sanofi Pasteur). All of these vaccines include inactivated forms of the virus and have been proven safe and effective, with long-lasting protection. None are licensed for children less than one year of age.
Twinrix (GlaxoSmithKline) is a combination vaccine used to prevent hepatitis A and hepatitis B infection. The name reflects the product’s composition as a mixture of two other vaccines (Havrix for HAV and Engerix-B for HBV). The vaccine works by causing the body to produce its own protection (antibodies) against these diseases. Twinrix Junior is available for children and adolescents aged 1 to 15 years.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | Vaqta® | Hepatitis A Pediatric | $14.25 | $30.37 |
| GlaxoSmithKline | Havrix® | Hepatitis A Pediatric | $14.25 | $28.74 |
| GlaxoSmithKline | Twinrix® | Hepatitis A-Hepatitis B 18 only | $47.50 | $89.85 |
The end date for these contracts is March 31, 2012.
Hepatitis B
Hepatitis B is a viral infection of the liver. Symptoms of the acute illness caused by the virus include liver inflammation, vomiting, jaundice, and occasionally, death. Chronic hepatitis B may also cause liver cirrhosis (replacement of liver tissue by fibrotic scar tissue) which may lead to liver cancer. Although up to two billion people, or one third of the world’s population, have been exposed to the virus, just 3% to 6% (195,000 to 390,000) are believed to be infected. Hepatitis B is preventable with a safe and effective vaccine.
The prevalence of chronic hepatitis B virus (HBV) infection is particularly high (more than 8%) in certain areas of the world. These include all of sub-Saharan Africa and most of Southeast Asia, including China, Indonesia, South Korea, and the Philippines; the Eastern Mediterranean except Israel; South and Western Pacific islands; the interior Amazon Basin; and certain parts of the Caribbean (the Dominican Republic and Haiti). The disease is moderately prevalent (2% – 7%) in South Central and South-West Asia, Israel, Japan, Eastern and Southern Europe, the Russian Federation, and most of Central and South America. In Australia, New Zealand, Northern and Western Europe, and North America, the prevalence of chronic HBV infection is low at less than 2% of the general population.
Universal infant immunization is now recognized as the proper strategy for every country for the long-term control of chronic HBV infection. The vaccine first became available in the United States in 1982 when the initial approach was to give it as pre-exposure vaccination to populations at high risk for HBV infection (e.g. healthcare workers, men who have sex with men, and heterosexual persons with multiple partners). Inoculation has benefited from the Advisory Committee on Immunization Practices’ recommendation of a comprehensive strategy to eliminate HBV transmission, including prevention of perinatal HBV transmission; universal vaccination of infants; catch-up vaccination of unvaccinated children and adolescents; and vaccination of unvaccinated adults at increased risk for infection. This has resulted in the U.S. incidence of acute hepatitis B declining by 75%, from 8.5 per 100,000 population in 1990 to less than 2.0 per 100,000 population in 2007, with the greatest declines (94%) among children and adolescents.
When the vaccine became widely available, a similar strategy was adopted by other industrialized countries. By the mid-1980s, several countries with very high prevalence of chronic HBV infection had begun routine immunization of infants at birth. The WHO subsequently recommended that hepatitis B vaccine be integrated into the national immunization program of all countries by 1997. As of December 2008, 177 countries have integrated the HBV vaccine into their routine infant immunization programs. The increased prevalence of HBV in the U.S. is attributed to a lack of concern about the severity of the disease and its threat. According to the CDC, all sexually active adolescents are at high risk for HBV. Because it is easier to immunize infants as part of the regular vaccination schedule than it is to convince teenagers to be vaccinated, CDC officials agree that shots for HBV should be included in all childhood immunization programs. Currently, it is estimated that less than 5% of the 28 million single Americans between the ages of 11 and 34 are vaccinated against HBV.
There are a number of recombinant hepatitis B vaccines on the world market including Recombivax HB (Merck); Engerix-B (GlaxoSmithKline); GenHevac B (Sanofi Pasteur); as well as Recvac-B and Recvac-Bmcf (Bharat Biotech). In 1996, Merck introduced Comvax, which is combination of the company’s PedvaxHIB, a prophylactic against Haemophilus influenzae type b and Recombivax HB vaccine.
It should also be noted, however, that some older HBV vaccines contained thimerosol, a highly toxic preservative that many believe increases the risk of autism. Although thimerosol has been largely phased out of vaccine manufacture, several manufacturers including Merck remain party to individual and class action product liability lawsuits involving pediatric hepatitis B vaccines that contained thimerosal. As of late 2009, there were approximately 200 thimerosal related lawsuits pending, most were not currently active.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| GlaxoSmithKline | Engerix B® | Hepatitis B Pediatric/Adolescent | $10.35 | $21.37 |
| Merck | Recombivax HB® | Hepatitis B Pediatric/Adolescent | $10.50 | $23.20 |
| GlaxoSmithKline | Twinrix® | Hepatitis A-Hepatitis B 18 only | $47.50 | $89.85 |
| Merck | Comvax® | Hepatitis B-Hib | $29.50 | $43.56 |
| GlaxoSmithKline | Pediarix® | DTaP-Hep B-IPV | $51.15 | $70.72 |
The end date for these contracts is March 31, 2012.
Measles, Mumps and Rubella
Safe and effective live-virus vaccines for measles, mumps and rubella have been available for over four decades. They are usually combined in one vaccine for children called the MMR vaccine, which is also recommended for adults born in 1957 or later. Persons born before 1957 are usually considered immune to measles, mumps, and rubella because most adults born during this time had these diseases in childhood.
This vaccine is available as a generic, produced by several manufacturers and available worldwide. Leading non-generic brands include:
- M-M-R II and ProQuad (Merck);
- Trimovax and R.O.R. (Sanofi Pasteur);
- Priorix (GlaxoSmithKline).
In September 2005, the FDA approved Merck’s ProQuad [Measles, Mumps, Rubella, and Varicella (Oka/Merck) Virus Vaccine Live]. ProQuad is a combination vaccine for simultaneous vaccination against measles, mumps, rubella and varicella in children 12 months to 12 years of age. ProQuad combines two established Merck vaccines, M-M-R II [Measles, Mumps, Rubella Virus Vaccine Live] and Varivax [Varicella Virus Vaccine Live (Oka/Merck)], thereby reducing the number of individual shots required for children by one. In early 2008, however, the CDC released findings of a study indicating that ProQuad doubles children's seizure risk compared with two separate MMR and chickenpox shots. Using ProQuad, 12- to 23-month-old children who get the vaccine have a 1 in 2,000 higher chance of having a febrile seizure—convulsions brought on by fever—than those getting two separate shots. Four out of every 10,000 children who got separate MMR and varicella shots on the same day had a febrile seizure seven to 10 days later, but nearly twice as many who got ProQuad—nine in 10,000— had febrile seizures seven to 10 days after vaccination. This resulted in the ACIP withdrawing its preference for ProQuad.
In August 2006, GlaxoSmithKline introduced Priorix-Tetra, a combination vaccine to prevent measles, mumps, rubella and varicella (MMRV), in Germany. The product’s rollout is proceeding slowly, however, amid controversy regarding testing. In March 2007, Russian prosecutors opened a criminal investigation to determine whether the vaccine’s clinical trials were conducted on children without parents' permission. As of mid 2010, Priorix-Tetra was available only in selected regions such as Canada and Australia.
Although a relatively large number of measles, mumps and rubella single vaccines were previously available, many of these have since been discontinued in favor of combination products.
Measles
Measles is a highly contagious viral disease that tends to appear in epidemics every two to three years and primarily affects children. Incubation lasts one to two weeks, after which symptoms resembling those of a cold develop, accompanied by high fever. Small red spots with white centers may appear on the inside of cheeks; on the third to fifth day, a blotchy, slightly elevated pink rash develops first behind the ears, then on the face, and then elsewhere. Patients remain infectious throughout the duration of the disease, and recovery typically takes two to four weeks.
Through the years, measles has exacted a heavy toll, killing an estimated 200 million persons over the past 150 years. In spite of available vaccination, measles remains a heavy public health burden worldwide. In the developing countries, measles may be ultimately responsible for more childhood deaths than any other single agent because of complications from pneumonia, diarrhea and malnutrition. Measles is also the major cause of preventable blindness in the world, affecting the same disadvantaged populations. According to the WHO, measles remains a leading cause of vaccine preventable childhood mortality with 30 million cases and hundreds of thousands of deaths worldwide caused by measles every year. However, the global immunization drive has cut measles deaths by 78% since 2000, with 164,000 deaths in 2008, according to the WHO. Of those, an estimated 90% were children who died before the age of five. From 2000 to 2008, almost 700 million children aged 9 months to 14 years who live in high risk countries were vaccinated against measles.
Of the deaths currently attributable to measles, 95% occur in developing countries, where vitamin A deficiency is common. India currently accounts for 67% of all measles deaths. Specific goals for reduction in measles mortality and morbidity were set by the World Heath Assembly in 1989 and the Word Summit for Children in 1990, as major steps towards the eventual eradication of the disease. Subsequently, the fourth Millennium Development Goal (MDG 4) aims to reduce the under-five mortality rate by two-thirds between 1990 and 2015. Recognizing the potential of measles vaccination to reduce child mortality, routine measles vaccination coverage has been selected as an indicator of progress towards achieving MDG-4.
It should also be noted, however, that measles have not yet been entirely eradicated in the developed nations, mainly due to voluntary refusal of immunization. In 2008, for example, the number of reported U.S. measles cases jumped to 140, the highest number since 1996. Of these, about half involved children whose parents rejected vaccination.
Mumps
Mumps, or parotis epidemica, is a viral infection primarily affecting the salivary glands. Although mostly a mild childhood disease, the mumps virus may also affect adults, among whom complications such as meningitis and orchitis are relatively common. Mumps orchitis occurs in 20% - 50% of post pubertal males. While both testes are affected in about 20% of these cases, mumps orchitis is rarely associated with permanently impaired fertility. However, a history of mumps orchitis seems to be a risk factor for testicular cancer. Encephalitis and permanent neurological sequelae are rare complications of mumps.
Until the advent of immunization, mumps had been a common infectious disease in all parts of the world, with annual incidences ranging from approximately 0.1% - 1.0%, and in certain populations reaching 6%. In hot climates, the disease is endemic throughout the year, whereas in temperate climates incidence peaks in late winter. Peak incidence is found among children five to nine years of age. Natural infection with mumps virus is likely to confer lifelong protection.
Nonetheless, mumps continues to occur even in the developed world. In 2006, the United States experienced a multi-state outbreak involving 6,584 reported cases of mumps, predominantly affecting Midwestern college students living in dormitories. Between 2004 and 2006, more than 70,000 mumps cases were reported in the U.K. As of early 2010, incidence in the U.K. remains relatively high at more than 4,000 cases and a mumps outbreak in New York and New Jersey between June 2009 and January 2010, affecting more than 1,500 persons, was traced to a young American who had visited the U.K. during the summer of 2009 then returned home.
Rubella
Rubella, also known as German measles, occurs worldwide and is normally a mild childhood disease. The name is derived from the Latin, meaning little red, and describes the rash which typically begins on the face, then spreads to the trunk and limbs and usually fades after about three days. Rubella usually occurs in a seasonal pattern (i.e. in temperate zones during the late winter and spring), with epidemics every five to nine years. However, the extent and periodicity of rubella epidemics is highly variable in both developed and developing countries. In early 2005, the U.S. Centers for Disease Control announced that rubella was no longer a public health threat in the United States.
Infection during early pregnancy may cause fetal death or congenital rubella syndrome (CRS); the latter characterized by multiple defects, particularly to the brain, heart, eyes and ears. CRS is a leading cause of hearing and visual impairment and mental retardation in countries where acquired rubella infection has not been controlled.
Although the burden of CRS is not well characterized in all countries, it is estimated that more than 100,000 cases occur each year in the developing countries alone. Caring for CRS cases is costly because of the permanent disabilities caused by this condition. Cost-benefit studies in developed as well as developing countries have demonstrated that, when combined with measles vaccine in countries with coverage of over 80%, the benefits of rubella vaccination outweigh the costs.
Current contract and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | MMRII® | Measles, Mumps and Rubella (MMR) | $18.99 | $50.16 |
The end date for the contract is March 31, 2012.
Meningococcal Disease
Meningococcal disease is a rare type of bacterial infection that typically causes meningitis. This disease represents an inflammation of the membranes (meninges) covering the brain, usually due to bacterial or viral infections elsewhere in body that has spread into the blood and into the cerebrospinal fluid (CSF). Symptoms typically include fever, headache, and neck stiffness. Approximately 10% of individuals who contract meningococcal disease die. Of those who survive, up to one in five suffer permanent disabilities such as hearing loss, neurological damage and limb amputations. Meningococcal disease often begins with symptoms that can be mistaken for the flu. But it can progress very rapidly and kill an otherwise healthy young person in 48 hours or less.
The highest incidence of meningitis in children is between birth and 2 years, with the greatest risk immediately following birth and at 3 - 8 months. Although vaccination has decreased incidence of meningitis considerably, parts of the developed world remain susceptible to outbreak. For example, large epidemics of meningococcal meningitis continue to occur in an area in sub-Saharan Africa which stretches from Senegal to Ethiopia and contains about 300 million people. The largest outbreak was in 1996, when over 250,000 cases occurred and 25,000 people died from the disease.
In October 2007, Sanofi Pasteur received FDA approval to expand the usage of its Menactra (Meningococcal [Groups A, C, Y and W-135] Polysaccharide Diphtheria Toxoid Conjugate Vaccine), to include children 2 years through 10 years of age. Menactra is the first and only quadrivalent conjugate vaccine licensed in the U.S. for the prevention of meningococcal disease. The vaccine first received FDA approval in 2005 for immunization of adolescents and adults 11 years through 55 years of age. Menactra offers protection against four of the five most common serogroups of the bacterium that cause meningococcal infection, Neisseria meningitidis serogroups A, C, Y and W-135. No vaccine is currently available in the United States for protection against infection from serogroup B.
In February 2010, Novartis received FDA approval for Menveo (MenACWY-CRM), a meningococcal serogroup C conjugate vaccine. The product is based upon the company’s Menjugate, a meningococcal serogroup C conjugate vaccine approved outside the United States since 2000 for use in persons from two months old through adulthood. In mid 2008, Novartis released Phase III trial data for Menveo suggesting that the product could become the first meningococcal vaccine to protect all age groups from infancy to adulthood against the four vaccine preventable serogroups (A, C, W-135 and Y). A pivotal Phase III study comparing Menveo with Menactra found that Menveo demonstrated statistically superior immune responses versus Menactra both on percentage of subjects protected and on the strength of the immune response. This was of special relevance for serogroup Y, which causes about 39% of meningococcal infections in the U.S. and where the usage of Menveo in this study increased the rate of protection of previously non-immune individuals by 50% compared to Menactra. At the end of 2009, Menveo was approved in the EU for prevention of disease in adults and children.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | Menactra® | Meningococcal Conjugate (Groups A, C, Y and W-135) | $82.12 | $106.49 |
| Novartis | Menveo® | Meningococcal Conjugate (Groups A, C, Y and W-135) | $82.12 | $106.49 |
The end date for these contracts is March 31, 2012.
Pneumococcal Disease
Streptococcus pneumoniae, or pneumococcus, is a leading cause of serious illness worldwide, and is the most frequent cause of pneumonia, bacteremia (infection of the blood), sinusitis, and acute otitis media (middle ear infection). Although all age groups may be affected, the highest rate of pneumococcal disease occurs in young children and in the elderly population. In addition, persons suffering from a wide range of chronic conditions and immune deficiencies are at increased risk.
Worldwide, more than 1.2 million children die each year as a result of pneumococcal diseases, according to the WHO. Ninety percent of these deaths occur in the developing world, but deaths also continue to occur in the developed nations. For example, the CDC reports that in the U.S. alone, Streptococcus pneumoniae causes approximately 17,000 infections per year among children under the age of 5, including 700 cases of meningitis and 200 deaths. Pneumococcal resistance to essential anti-microbials such as penicillins, cephalosporins and macrolides is a serious and rapidly increasing problem worldwide.
The pneumococcal conjugate vaccine Prevnar (Pfizer) was FDA-approved for use in children in 2000 in the United States, and is now approved in more than four dozen countries. The vaccine - pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein containing 4,6B,9V,14,18C,19F, and 23F) - targets the seven serotypes of pneumococcal bacteria most prevalent in the U.S., which are also among the most resistant to antibiotics and cause 80% of pneumococcal disease in infants. The vaccine is 97% protective against invasive pneumococcal disease caused by vaccine serotypes in fully vaccinated children. Prevnar is currently being recommended for children only, but studies in adults are ongoing.
In February 2010, Pfizer received FDA approval for a new 13- valent conjugate vaccine, which protects against the 13 most prevalent serotypes associated with pneumococcal disease. Seven of these serotypes are included in Prevnar; the six additional serotypes (1, 3, 5, 6A, 7F and 19A) are associated with the greatest remaining burden of invasive disease and particularly address virus types common in the developing countries. Both Prevnar 13 and Prevnar use CRM197, an immunological carrier protein.
In the U.S., the American Association of Pediatrics (AAP) recommends the routine use of Prevnar for all children 23 months and younger, and for children aged 24 to 59 months who are at high risk, including children with an immune deficiency, sickle cell disease, asplenia (children without a working spleen), HIV infection, chronic cardiac conditions, chronic lung problems (including asthma), cerebrospinal fluid leaks, chronic renal insufficiency (including nephrotic syndrome) , diabetes mellitus, and children who are receiving immunosuppressive therapy (organ transplants, etc.). This has resulted in a significant decrease in incidence of VT IPD among children under five, with cases declining from 80 per 100,000 population in 1998 to less than 3 per 100,000 population in 2009. The AAP does not recommend routine use in children who are only at moderate risk of infection, including children aged 24 to 35 months old, and children aged 36 to 59 months who attend daycare or who are of Native American, Alaskan native, or African American descent.
In January 2009, GlaxoSmithKline’s new Synflorix received a positive opinion from the European Medicines Agency (EMEA). for active immunisation against invasive pneumococcal disease (IPD) and middle ear infections (acute otitis media) caused by Streptococcus pneumoniae in infants and children from 6 weeks up to 2 years. The 10-valent vaccine incorporates a new approach in pneumococcal vaccine technology, as it is designed with an active carrier protein to induce protection against non-typeable Haemophilus influenzae (NTHi) in addition to Streptococcus pneumoniae.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Pfizer | Prevnar 13 TM | Pneumococcal 13-valent(Pediatric) | $97.21 | $114.15 |
| Merck | Pneumovax® | Pneumococcal Polysaccharide (23 Valent) | $34.54 | $56.31 |
The end date for these contracts is March 31, 2012.
Polio
Polio (poliomyelitis) is an infectious viral disease that affects the central nervous system (CNS). It is transmitted through the respiratory tract, by the fecal-oral route, or from mother to infant at the time of birth. The poliovirus initially invades the throat and intestines, and may then move to the lymphoid tissue, bloodstream, or CNS. Most polio patients are asymptomatic, but those with CNS involvement may develop paralysis. Since polio immunizations were introduced in the 1960s, they have successfully eradicated poliomyelitis in many parts of the world. This has been due in large measure to the Global Polio Eradication Initiative, a program spearheaded by the WHO and the CDC which represents the largest public health initiative in the history of the world.
There are still many countries, however, in which polio occurs. Nigeria and India have the highest absolute numbers of polio cases, reporting 559 and 798 cases in 2008, respectively. Other countries in which polio continues to be endemic include Pakistan, with 117 cases in 2008 and Afganistan, with 31 cases. Polio cases due to active transmission continue to occur in Angola, Burkina Faso, Benin, the Central African Republic, Chad, Côte d'Ivoire, the Democratic Republic of the Congo, Ethiopia, Ghana, Mali, Nepal, the Niger, the Sudan and Togo.
Despite good immunization coverage in many of these areas, studies have shown that one of the main reasons for the high persistence of the disease is overcrowded living conditions and poor sanitation. Those conditions in the Indian provinces of Uttar Pradesh and Bihar, for example, have made the oral poliovirus vaccine less effective than in other parts of India and immunized children are still being infected. This occurs as the three strains in the trivalent vaccine can interfere with each other inside the body, producing immunity to one strain but not another. The researchers suggest switching to a monovalent vaccine against the dominant strain in these regions from the standard trivalent vaccine that protects against three types of polio virus. The most successful strategy, argues the study, would be to first address the type 1 strain of the virus, which is the most prevalent worldwide, and inoculating against the type 3 strain.
There are two types of polio vaccines available: an oral live virus vaccine (OPV), and inactivated poliovirus (IPV), which is a killed vaccine that is administered by injection. Sanofi Pasteur manufactures IMOVAX Polio, an IPV vaccine that was chosen in November 2008 by the Russian government for primary immunization of all infants. Novartis offers IPV-Virelon, Polioral and TD-Virelon. In India, Bharat Biotech sells Biopolio.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | IPOL® | e-IPV | $11.97 | $25.43 |
| GlaxoSmithKline | Kinrix® | DTaP-IPV | $34.25 | $48.00 |
| GlaxoSmithKline | Pediarix® | DTaP-Hep B-IPV | $51.15 | $70.72 |
The end date for these contracts is March 31, 2012.
Rotavirus
Rotavirus (RV) infection is the leading cause of severe diarrhea and vomiting in infants and young children between 3 - 24 months worldwide. If untreated, the virus can rapidly kill, as the sickest children become dehydrated from 10 to 20 episodes of diarrhea in a single day.Globally, rotaviruses account for approximately 138 million cases of infantile gastroenteritis each year. According to the WHO, RV gastroenteritis causes 70,000 or more hospitalizations a year in the United States alone, in addition to a half-million physician and clinic visits, and 20 to 40 deaths. Worldwide, RV is estimated to account for almost 40% of all cases of severe diarrhea, which translates into 600,000 deaths each year. Up to 85% of these deaths occur in countries defined as “low-income” according to the World Bank classification scheme. These represent countries with less than $975 gross national income per capita in 2008 and include Afghanistan, Bangladesh, Benin, Bhutan, Burkina Faso, Burundi, Cambodia, Central African Republic, Chad, Comoros, Congo, Eritrea, Ethiopia, Gambia, Ghana, Guinea, Guinea-Bisau, Haiti, Kenya, Korea, Kyrgyz Republic, Lao, Liberia, Madagascar, Malawi, Mali, Mauritania, Mozambique, Myanmar, Nepal, Niger, Rawanda, Senegal, Sierra Leone, Somalia, Tajikistan, Tanzania, Togo, Uganda, Uzbekistan, Vietnam, Yemen, Zambia and Zimbabwe.
In epidemiological studies conducted in developing countries, RV accounted for about 8% of all diarrhea episodes, 28% of outpatient visits for diarrhea, and 34% of hospitalizations of young children for diarrhea. In Peru, for example, RV is accountable for 384,000 cases, 64,000 clinic visits, 30,000 hospitalizations, and 1,600 deaths per year with a $2.6 million medical care cost. RV is responsible for 25% of the deaths associated with diarrhea and responsible for 6% of all deaths in children less then 5 years of age.
RV is also present in the developed nations, although it is considerably less deadly. Over 3 million cases of RV gastroenteritis occur annually in the U.S. resulting in the hospitalization of approximately 55,000 children each year. However, just 20 to 40 deaths each year are attributed to RV in the U.S. In temperate areas, it occurs primarily in the winter, but in the tropics it occurs throughout the year.
Only a handful of rotovirus vaccines are currently licensed. Rotarix (GlaxoSmithKline/Avant) was approved by the Board of Health in Mexico in July 2004 for the prevention of gastroenteritis caused by rotavirus infection and was subsequently approved in the E.U. Rotarix is an oral, two-dose, live attenuated vaccine developed from a single human strain designed to provide broad protection against multiple rotavirus strains. In April 2008, the FDA approved Rotarix for sale in the United States, based on data from almost 75,000 infants. Clinical data show that Rotarix provides protection through the first two years of life and is highly efficacious against rotavirus hospitalizations (96%), severe rotavirus gastroenteritis (90%) and rotavirus gastroenteritis of any severity (79%). Specifically, significant protection was demonstrated against severe rotavirus gastroenteritis caused by types G1 (96%), G2 (86%), G3 (94%), G4 (95%), and G9 (85%), the most commonly circulating rotavirus types in the U.S.
In February 2006, Merck’s RotaTeq was approved by the FDA. RotaTeq is a live, oral pentavalent vaccine that contains 5 live reassortant rotaviruses. It is indicated for the prevention of rotavirus gastroenteritis in infants and children caused by the serotypes G1, G2, G3, and G4 when administered as a 3-dose series to infants between the ages of 6 to 32 weeks, with a first dose administered between 6 and 12 weeks of age. Since its introduction, more than 10 million doses of RotaTeq have been distributed.
In 2010, several global regulators issued a warning regarding usage of Rotarix, then revised recommendations against the vaccine’s uage. In March, the U.S. FDA followed the European Medicines Agency (EMEA) and Swissmedic, advising pediatricians to stop using Rotarix vaccine after independent researchers found porcine circovirus 1 (PCV1) in the product. The PCV1 virus is not known to cause illness, however, vaccines are required to be sterile and the presence of the virus is unexpected in the product. In May 2010, the FDA announced revised recommendations, determining that it is appropriate for health care professionals to resume the use of Rotarix and to continue the use of RotaTeq.
Other developers of a rotavirus vaccine include India-based Bharat Biotech, which in 2009 began Phase III trials of its 116E vaccine candidate. The studies will be mainly in India and enroll 6,800 healthy infants.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | RotaTeq® | Rotavirus, Live, Oral, Pentavalent | $59.76 | $69.59 |
| GlaxoSmithKline | Rotarix® | Rotavirus, Live, Oral, Oral | $89.25 | $102.50 |
The end date for these contracts is March 31, 2012.
Varicella
Varicella (chicken pox) is an acute, highly contagious viral disease caused by varicellazoster virus (VZV). It is characterized by a rash of itchy, inflamed lesions. The disease has a 10 - 21 day incubation period and is highly contagious through physical contact two days before symptoms appear. Following primary infection there is usually lifelong protective immunity from further episodes. While mostly a mild disorder in childhood, varicella tends to be more severe in adults. It may be fatal, especially in neonates and in immunocompromised persons. Following infection, the virus remains latent in neural ganglia, and upon subsequent reactivation VZV may cause zoster (shingles), a disease mainly affecting the elderly and immunocompromised persons. Although individual cases may be prevented or modified by varicella-zoster immune globulin or treated with antiviral drugs, control of varicella can be achieved only by widespread vaccination.
Varicella vaccines based on the attenuated Oka-strain of VZV have been marketed since 1974, and their positive results have warranted the introduction of these vaccines into the childhood immunization programs of several industrialized countries. After observation of study populations for periods of up to 20 years, more than 90% of immunocompetent persons who were vaccinated as children were still protected from varicella. A generic form of the varicella vaccine is available from various manufacturers worldwide. Brand names include Varivax (Merck) and Varilrix (GlaxoSmithKline).
Current contract and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | Varivax® | Varicella | $69.73 | $83.77 |
The end date for the contract is March 31, 2012.
Adult Vaccine
Diphtheria, Tetanus
Diphtheria and tetanus are serious, but distinctly different, diseases. Beginning in childhood, vaccinations for both tetanus and diphtheria (TD) are included in combination vaccines, DTP and DTaP. This practice continues into adulthood, when TD vaccines usually are administered together, minus the pertussis. The exception is when tetanus vaccine may be given alone as part of wound management.
Diphtheria is an acute respiratory tract infection that is caused by Corynebacterium diphtheriae. There are several forms, including membranous pharyngotonsillar diphtheria, nasal diphtheria, obstructive laryngotracheitis, and cutaneous diphtheria. Diphtheria is rare, but poorly immunized adults are among the most affected populations. The incubation period is two to five days. Diphtheria outbreaks tend to occur in the fall and winter in temperate regions, but such trends are less distinct in the tropics. The cutaneous form peaks between August and October in the southern United States.
Tetanus is a severe disease that affects the central nervous system. It is caused by Clostridium tetani, an organism that grows in soil and in the intestinal tracts of humans and animals. The mode of transmission is animal-to-human via wounds, including punctures, surgical incisions, or burns. The incubation period is about eight days, with a three-day to three-week range. Risk factors for the disease include lack of primary immunization and inappropriate wound care. Because of this, adults aged 60 years and older are at the highest risk for tetanus, particularly in the developing nations.
Leading products include a variety of vaccines from Novartis (Tetanol, Tetanol pur, Adsorbed Diphtheria Vaccine Behring for Adults, Td-pur, Td-Virelon, Td Vaccine Behring and Dif-Tet-All) and Sanofi-Aventis (Pentacel, Quadracel, Adacel, Td Adsorbed, Td Polio Adsorbed). Although inoculations are most commonly administered in childhood, they are also recommended for adults who have not been vaccinated.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | Adacel® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $26.25 | $38.83 |
| GlaxoSmithKline | Boostrix® | Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis | $26.25 | $37.55 |
| Sanofi Pasteur | Decavac® | Tetanus and Diphtheria Toxoids | $13.82 | $20.39 |
The end date for these contracts is June 30, 2011.
Hepatitis A
Hepatitis A virus (HAV) causes a contagious viral infection that is contracted from contaminated food or water and is transmitted most often by the fecal-oral-route. If persons incubating the disease donate blood or plasma, HAV may be transmitted to subsequent recipients, although transmission in this manner is rare.
About a quarter of those infected with HAV have no symptoms at all, but others typically experience fatigue, fever, abdominal pain, nausea, diarrhea, loss of appetite, depression and jaundice. There is no specific treatment for hepatitis A. Sufferers are advised to rest, avoid fatty foods and alcohol, eat a well-balanced diet, and stay well hydrated. Approximately 15% of people diagnosed with hepatitis A may experience a symptomatic relapse for nine months to a year after contracting the disease.
Groups at greatest risk for hepatitis A include intravenous drug users, children and workers in day-care centers, international travelers, military personnel, food handlers, male homosexuals, sewage workers, health-care workers, closed-institution occupants, those in contact with HAV-infected persons, and individuals with chronic liver disease. In countries highly endemic for hepatitis A, almost all persons are infected in childhood without showing symptoms, effectively preventing clinical diagnosis of hepatitis A in adolescents and adults. In these countries, large-scale vaccination programs are not recommended. In countries of intermediate disease endemicity, where a relatively large proportion of the adult population is susceptible to HAV, and where hepatitis A represents a significant public health burden, large-scale childhood vaccination may be considered as a supplement to health education and improved sanitation.
In regions of low disease endemicity, vaccination against hepatitis A is indicated for individuals with increased risk of contracting the infection, such as travelers to areas of intermediate or high endemicity.
Several inactivated vaccines against HAV are internationally available. All are safe and effective, with long-lasting protection. Included among the HAV products on the market are Avaxim and ViVAXIM (Sanofi Pasteur), Epaxal (Crucell), Havrix (GlaxoSmithKline), HAVpur (Novartis) and Vaqta (Merck). As of early 2010, Epaxal was the only aluminium-free hepatitis A vaccine on the market, thereby offering greater tolerability for some persons than other products. The first product to be based on the virosome technology developed by Crucell, it induces protective antibody levels within 10 days of primary vaccination, and provides seroprotection for at least 20 years following the second dose. GSK also produces a combination vaccine, Twinrix, which includes hepatitis A vaccine. This product is only approved for persons of 18 years and older. However, a new product Twinrix Junior, has become available for HAV and HBV vaccinations in children.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| GlaxoSmithKline | Havrix® | Hepatitis A Adult | $21.59 | $63.1 |
| GlaxoSmithKline | Twinrix® | Hepatitis A-Hepatitis B Adult | $43.5 | $89.85 |
The end date for these contract is June 30, 2011.
Hepatitis B
Hepatitis B (HBV) infection is one of the leading causes of liver disease worldwide. More than 2 billion people alive today have been infected with the HBV virus. The disease is largely transmitted through exposure to bodily fluids containing the virus, including unprotected sexual contact, blood transfusions, re-use of contaminated needles and syringes, transmission from mother to child during childbirth, etc. Approximately 350 million are chronically infected and are at high risk of serious illness and death from cirrhosis of the liver and primary liver cancer, diseases that kill 500,000 to 750,000 persons a year. Hepatitis B is currently the second most prevalent cause of cancer in humans after tobacco smoke, yet is preventable through vaccination.
The prevalence of chronic HBV infection is particularly high (more than 8%) in certain areas of the world. These include all of sub-Saharan Africa, South-East Asia, including China, Indonesia, the Democratic People’s Republic of Korea, and the Philippines; the Eastern Mediterranean except Israel; South and Western Pacific islands; the interior Amazon Basin; and certain parts of the Caribbean (the Dominican Republic and Haiti). The disease is moderately prevalent (2% to 7%) in South Central and South- West Asia, Israel, Japan, Eastern and Southern Europe, the Russian Federation, and most of Central and South America. In Australia, New Zealand, Northern and Western Europe, and North America, the prevalence of chronic hepatitis B viral infection is low (under 2% of the general population). However, infection can still occur, such as with the June 2010 announcement that almost 2,000 patients at one free dental clinic in West Virginia had been exposed to hepatitis B and several patients and volunteers at the clinic had contracted the disease.
Included among the many HBV vaccine products on the market are Engerix-B (GlaxoSmithKline), Hepavax-Gene (Crucell), Nabi-HB (Biotest), and Recombivax HB and Comvax (Merck). However, these products are not ideal for certain populations, such as immunocompromised patients. To a limited extent, research into new products continues. For example, in November 2007, Dynavax and Merck announced a global license and development collaboration agreement to jointly develop Heplisav, a novel Toll-Like Receptor 9 agonist HBP vaccine, being evaluated in a multi-center Phase III clinical trial involving patients on dialysis. In mid 2008, however, the project was put on hold indefinitely.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| GlaxoSmithKline | ENGERIX-B® | Hepatitis B-Adult | $28.00 | $52.50 |
| GlaxoSmithKline | Twinrix® | Hepatitis A-Hepatitis B Adult | $43.50 | $89.85 |
The end date for these contracts is June 30, 2011.
Human Papilloma Virus
Cervical cancer is the sixth most frequent malignancy in women, behind cancer of the breast, colon, rectum, endothelium, lung and ovary. It occurs predominantly in women between 30 and 50 years of age. The number of deaths from cervical cancer has fallen steadily over the past 40 years due to better and earlier diagnosis with the widespread use of the Pap test. However, there is currently a resurgence of cervical cancer in young women, particularly in the United States. Risk varies directly with an increase in the number of sexual partners, early incidence of first intercourse and exposure to certain serotypes of papilloma virus (HPV), the most common sexuallytransmitted infection in the U.S. On average, 9,710 new cases of cervical cancer and 3,700 deaths are attributed to HPV in the U.S. each year. Worldwide, cervical cancer is the second most common cancer in women, with 470,000 new cases annually and 233,000 deaths. HPV infection affects about 20 million people in the U.S., with 6.2 million new cases each year.
In June 2006, the FDA approved Merck’s Gardasil, a new preventive vaccine that can reportedly prevent up to 70% of the 10,000 cases of cervical cancer diagnosed in the United States each year. The vaccine was developed to target four strains of HPV, two of which (HPV 16 and 18) are linked to cervical cancer and two (HPV 6 and 11) which cause anogenital warts. It was initially approved for use in females ages 9 to 26, and in September 2008, the FDA approved Gardasil for the prevention of vulvar and vaginal cancers caused by HPV types 16 and 18. A subsequent approval in October 2009 allowed Gardasil to be marketed for the prevention of genital warts due to HPV types 6 and 11 in boys and men ages 9 to 25.
Merck set an initial price of $120 a shot, making Gardasil one of the costliest vaccines available. The vaccine is administered in three doses over six months. It is genetically engineered and does not contain live viruses that could theoretically cause disease. The vaccine is known to be effective for at least 3 1/2 years and may need a booster after that. As of mid 2010, Gardasil has been approved in more than 100 countries including the European Union, Australia, New Zealand, Canada, Mexico, Croatia, Malaysia, Brazil, Serbia and Israel, with further approvals pending. It should also be noted, however, that while studies to date have shown the vaccine to be largely without side effects, some concerns have been raised. In February 2009, Spanish authorities withdrew tens of thousands of doses of vaccine afater two teenagers who received the shots were hospitalized; this prompted Singapore officials to call for caution in carrying out mass immunizations, saying the vaccine should be studied further.
In collaboration with several developers, particularly Australia-based CSL, Merck is currently in Phase III studies of another cervical cancer vaccine, V503, that targets nine HPV subtypes: 6, 11, 16, 18, 31, 33, 45, 52 and 58. It would therefore confer the protection of Gardasil, along with protection against another five HPV subtypes.
Cervarix, offered by GlaxoSmithKline, also protects against two strains of HPV (16 and 18) that cause cervical cancer. In 2007, the drug was launched in selected regions including Australia and the Philippines and in October 2009, the U.S. FDA approved Cervarix. At the same time, Cervarix was approved in Japan, becoming the first HPV vaccine available in that country.
Despite strong sales, utilization of the vaccines remains controversial, with some parents believing that vaccinating their daughters would encourage sexual activity. Others, however, believe that widespread vaccination would diminish the presence of HPV in the population and have advocated mandatory vaccination programs. Table 4-3 shows U.S. state initiatives as of early 2010.
It should also be noted, however, that future indications for these products could include other types of cancer as research has established a link between HPV and other mucosal cancers such as head and neck cancers, cancers in the urinary tract and genital region; tonsils, pharynx, the digestive tract; and vulva, penis, vagina and anal cancer.
Current contracts and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | Gardasil® | HPV -Quadrivalent Human Papillomavirus Types 6, 11, 16 and 18 Recombinant Adult | $89.17 | $130.27 |
| GlaxoSmithKline | Cervarix® | HPV-Human Papillomavirus Bivalent Types 16 and 18 | $85.64 | $128.75 |
Pneumococcal Disease
Infections caused by pneumococci are a major cause of morbidity and mortality all over the world. Pneumonia, febrile bacteraemia, and meningitis are the most common manifestations of invasive pneumococcal disease. Bacterial spread within the respiratory tract may result in middle-ear infection, sinusitis, or recurrent bronchitis. Compared with invasive disease, the non-invasive manifestations are usually less severe but considerably more common.
In developing countries, at least one million children die of pneumococcal disease every year. In the developed world, however, elderly persons carry the major disease burden. Conditions associated with increased risk of serious pneumococcal disease include HIV infection, sickle-cell anemia and a variety of chronic organ failures. In the United States, 7 million cases of otitis media (middle ear infection) are attributed to pneumococci each year. Because of this, inoculation continues to be recommended for at risk groups. In the U.K., for example, pneumococcal vaccination is recommended as a part of routine vaccination schedules for those over the age of 65, and also for both children and adults in special risk categories, such as those with difficulty breathing, serious heart conditions, severe kidney problems and chronic liver disease.
Pneumococcal vaccines for adults available worldwide include Pneumovax 23 from Merck. The polyoside vaccine contains 23 different serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F) covering 90% of the serotypes incriminated in invasive infections. Wyeth (which is now part of Pfizer) ceased producing its Pnu-Immune in 2002.
Intercell is developing a novel vaccine targeting a majority of the subtypes of the pneumococcus bacteria. The vaccine is intended primarily for children in developing countries, although it is also expected to be used widely by older persons in developed countries. A Phase I clinical trial initiated in 2009 indicated good safety and tolerability of the vaccine candidate.
It should be noted, however, that a mid 2010 study conducted in Canada and published in Clinical Infectious Diseases revealed that pneumococcal vaccine was unable to prevent repeat episodes of pneumonia. The study found that half of patients hospitalized for pneumonia die or return to hospital within five years from another bout of pneumonia, whether or not they receive the vaccine. Researchers postulate that these results are valid since clinical trials for the vaccine were conducted on younger patients with stronger immune systems.
Current contract and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | Pneumovax® | Pneumococcal Polysaccharide (23 Valent) | $18.73 | $43.63 |
The end date for the contract is June 30, 2011.
Shingles
Shingles, also known as herpes zoster (or zoster), is a viral disease characterized by a painful skin rash with blisters in a limited area of the body. It is caused by varicella zoster virus (VZV), which is also the virus that causes chickenpox. After a varicella infection, the virus can lodge permanently in ganglionic neurons, or sometimes in the non-neuronal satellite cells, without causing any symptoms. In an immunocompromised individual, the virus may break out of nerve cell bodies and travel down nerve axons to cause viral infection of the skin in the region of the nerve. This can occur years or even decades after the initial chickenpox infection. Although the rash usually heals within two to four weeks, and healing is furher accelerated with antiviral drug treatment, some sufferers experience residual nerve pain for months or years (postherpetic neuralgia). Each year, shinges affects approximately 500,000 persons in the U.S. and 10 million people worlwide.
The first, and thus far only, vaccine designed to prevent against shingles is Merck’s Zostavax, approved by the FDA in May 2006. Zostavax is a live vaccine that was shown to reduce the incidence of shingles by 51.3% in a pivotal phase III study of 38,000 adults aged 60 and older who received it. The vaccine also reduced by 66.5% the number of cases of postherpetic neuralgia and reduced the severity and duration of pain and discomfort associated with shingles, by 61.1%. In October 2006, the ACIP voted to recommend that Zostavax be given to all adults age 60 and over, including those who have had a previous episode of shingles. Merck is also reportedly testing Zostavax for use among 50 to 59 year olds.
Vaccination is expected to both improve the quality of life and reduce health care costs associated with shingles. A study published in an October 2007 issue of Vaccine projects an annual savings of $82 to $103 million in U.S. healthcare costs. Because of this, a rising number of insurers now cover vaccination with Zostavax. However, sales remain relatively low at just $277.4 million in 2009, with an 11% decline from the prior year.
This was the result of product shortages due to unexpectedly high demand and ingredient supply disruptions. During late 2008 and early 2009, high demand for the product resulted in waiting lists for many patients. Contributing to the shortage was the fact that Zostavax requires the same base ingredient as varicella vaccine, which had been in short supply, and the CDC had recently increased its recommendation of varicella shots from one to two.
Reimbursement fees also contributed to declining usage, with a 2010 survey of 600 physicians finding that 12% have stopped offering the Zostavax vaccination since Medicare does not always cover the $200 cost; as a result, only 41% of physicians surveyed strongly recommended Zostavax to their patients.
Current contract and pricing
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Merck | Zostavax® | Zoster Vaccine Live | $105.94 | $153.93 |
The end date for the contract is June 30, 2011.
Influenza Vaccine
The influenza vaccine protects against three influenza viruses that research indicates will be most common during the upcoming season. The 2010-2011 flu vaccine will protect against 2009 H1N1, and two other influenza viruses (an H3N2 virus and an influenza B virus). The viruses in the vaccine change each year based on international surveillance and scientists' estimations about which types and strains of viruses will circulate in a given year.
The vaccine should be taken each year in September or as soon as vaccine is available and continue throughout the influenza season. This is because the timing and duration of influenza seasons vary. While influenza outbreaks can happen as early as October, most of the time influenza activity peaks in January or later.
On February 24, 2010 vaccine experts voted that everyone 6 months and older should get a flu vaccine each year starting with the 2010-2011 influenza season. CDC's Advisory Committee on Immunization Practices (ACIP) voted for "universal" flu vaccination in the U.S. to expand protection against the flu to more people.
There are two types of vaccines:
- The "flu shot" — an inactivated vaccine (containing killed virus) that is given with a needle, usually in the arm. The flu shot is approved for use in people older than 6 months, including healthy people and people with chronic medical conditions.
- The nasal-spray flu vaccine —a vaccine made with live, weakened flu viruses that do not cause the flu (sometimes called LAIV for "live attenuated influenza vaccine"). LAIV is approved for use in healthy* people 2-49 years of age who are not pregnant. FluMist, manufactured by MedImmune is the only nasal-spray flu vaccine approved in USA.
Companies and Products
The following companies have currently been awarded contracts to supply vaccines in USA.
| S. No. | Company | Product Brandname | Age restrictions |
| 1 | GlaxoSmithKline | Fluarix | 36 months and older |
| 2 | GlaxoSmithKline | FluLaval | 18 years and older |
| 3 | MedImmune | FluMist | 2 - 49 years |
| 4 | Merck (CSL product) | Afluria | 9 years and older |
| 5 | Novartis | Fluvirin | 4 years and older |
| 6 | Sanofi Pasteur | Fluzone | 6 months and older |
The end date for these contracts is February 29, 2012.
Source: [5]
Vaccine Pricing
Influenza vaccines use thimerosal as a preservative. The price of the vaccine increases if it is free of preservatives.
Prices for vaccines containing thimerosal as a preservative:
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| GlaxoSmithKline | FluLaval ® | Influenza (Age 18 years and older) | $7.48 | $7.83 |
| Sanofi Pasteur | Fluzone® | Influenza (Age 6 months and older) | $9.30 | $11.17 |
| Novartis | Fluvirin® | Influenza (Age 4 years and older) | $10.70 | $12.10 |
| Merck (CSL poduct) | Afluria® | Influenza (Age 9 years and older) | $8.25 | $10.25 |
Prices of vaccines free of preservatives:
| Manufacturer | Brandname/ Tradename | Vaccine Details | CDC Cost/Dose | Private Sector Cost/Dose |
| Sanofi Pasteur | Fluzone® Pediatric dose | Influenza (Age 6-35 months) | $11.68 | $13.16 |
| Sanofi Pasteur | Fluzone® | Influenza (Age 36 months and older) | $10.97 | $12.41 |
| GlaxoSmithKline | Fluarix® | Influenza (Age 36 months and older) | $8.90 | $10.98 |
| Novartis | Fluvirin® | Influenza (Age 4 years and older) | $12.75 | $13.60 |
| Merck (CSL product) | Afluria® | Influenza (Age 9 years and older) | $9.00 | $11.00 |
| MedImmune | FluMist® | Influenza Live, Intranasal (Age 2-49 years) | $15.70 | $19.70 |
Source: [6]
Vaccines Market in United States
Characteristics of US market
Like any other vaccine market US vaccine market is can be considered as monopsony type market with Government being sole biggest purchaser of vaccine influencing dynamics of Vaccine Market
Entire Vaccine Sale in USA can be broken down in 4 segments
| Entity | Type | Sales Share |
| Hopsitals, Physicians | Private | 43% |
| The VFC (Vaccines For Children) program: | Government | 41% |
| Immunization Grant Program (Section 317): | Government | 11% |
| State/local governments: | Government | 5% |
As can be seen from the table government forms more than half of the share of vaccine sales
Dynamics of Government Market of Vaccines
General Policy of Awarding Contracts
Prior to 1993, the CDC used a winner-take-all strategy, awarding all sales to the lowest bidder. This resulted in low prices and great uncertainty for suppliers. Since 1998, the CDC solicits bids annually but does not directly purchase vaccines. Rather, it posts bid prices of firms with which it has negotiated contracts, usually with a near-zero minimum and a negotiated maximum quantity, and bidders can adjust prices quarterly. State and local grantees that receive budgets for vaccine purchase decide which suppliers to use. States may also purchase vaccines at CDC prices for non-VFC programs that are federally authorized, including using their own funds to supply all vaccines used within the state (" universal purchase"). Vaccines with federal contracts in 1993 are subject to a cap on price increases equal to the Consumer Price Index (CPI). This created incentives to develop new variants of existing vaccines such as combination products which are not subject to the price cap.
Prices Offered by Government
http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm
Current State of Competition
In 1967 there were 26 licensed manufacturers, but only 12 in 2002. Five firms produce almost all routine childhood vaccines, with a sole supplier for five of the eight recommended pediatric vaccines.1 When key suppliers experience manufacturing problems, supply interruptions and vaccine shortages interrupt immunization schedules, sometimes leading to children not being immunized.
Vaccine Approval Process
| FDA Approval Times | |
| Type of Vaccine | Mean Time in Duration |
| Innovative Vaccine | 13.9 |
| Follow-on Vaccine | 33.3 |
GlaxoSmithKline profile
Assessment of Various Entry methods for Serum India
Objective of the report is to find suitable partnership entry option. Partnerships can be at different stages.
Product-Channel Partnership Technology-Production Partnership License-Production Partnership
Strengths and Weaknesses of Serum Institute of India
Strengths
i) Manufacturing Capabilities: Ability to manufacture range of vaccines on a large scale efficiently.
ii) Exporting Vaccine products to 139 countries as of now.
Weakness
i) No brand value in US market
ii) No prior experience of operations in US market which is vital due to its tough regulatory environment
To enter into US market, Serum Institute of India will have to enter into partnership in such way as to build on its strengths and cover weaknesses.
Best suited option for Serum India is to handle manufacturing of vaccines by manufacturing low cost vaccines in India and licensing market operations to partnership firm which should take care of i) Approval ii) Distribution iii) Marketing. Partnership firm needs to have experience of operations in bio-tech sector in United States.