Enhanced bioavailability of skin whitening actives in topical applications
Contents
Objective
To understand different strategies used for enhancing the bioavailability of skin whitening agents in topical applications by various companies.
Scope
- This report is restricted to four major players in this technology area- Kao, L'Oreal, P&G and Shiseido.
- We have taken patents as a source of information to understand different methods for enhancing bioavailability.
Search methodology
| Search strategy | 1. Various keywords were retrieved for conducting the search related to enhanced bioavailability of active ingredients in topical applications for skin whitening from pubmed MESH, relevant patents, scientific articles and thesaurus. 2.Various Class-codes have been retrieved from relevant patents and their citations. Also . (Refer section 4). 3.The database used for patent search is Thomson innovation. |
| Keywords | Whitening, lightening, topical, depigmentation, bioavailability, penetration enhancer etc. |
Introduction
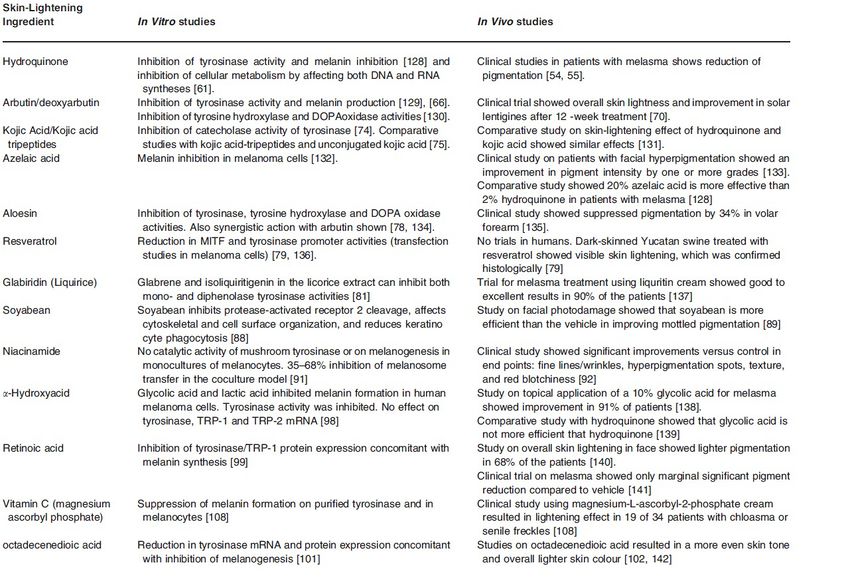
Skin whitening/lightening/brightening is an important skin care need, especially in the asian population. Whitening skin care is a process in which the darker skin is prepared to look lighter. Human skin color determined from the outermost layer of the skin, the epidermis where the pigment-producing cells melanocytes are localized to produce melanin. The amount of melanin synthesized by the melanocyte, and its distribution pattern in the surrounding keratinocytes, determines the actual color of the skin. Melanin forms through a series of oxidative reactions involving the amino acid tyrosine in the presence of the enzyme tyrosinase.Parvez et al,2006
Elimination of melanin can be done in three ways:
- Suppressing Tyrosinase formation
- Inhibiting Tyrosinase activity
- Reduction of Melanin
Hydroquinone and hydroxyanisole, in the past, were the standard ingredient for skin lightening treatments. However with reports of potential mutagenicity, there has been an increasing impetus to find alternative herbal and pharmaceutical depigmenting agents.
Current trends in skin whitening ingredients
Great advances have been made to understand pigment biology and the processes underlying skin pigmentation in the last decade. Many researchers have begun to produce natural alternatives which mimic the skin lightening properties of hydroquinone and hydroxyanisole. Ingredients such as kojic acid and licorice have become quite popular along with more advanced ingredients like Alpha-Arbutin. When combined, these ingredients can often produce better results that even surpass hydroquinone but without the associated risks. Below is the list of commonly used ingredients that are widely used for skin whitening.
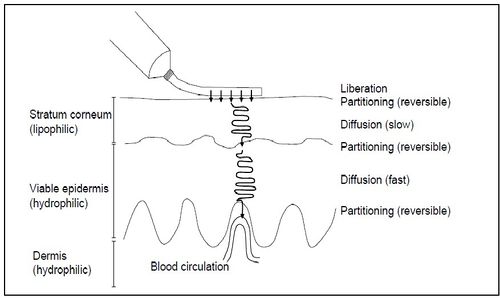
- Because of the accessibility and large area of the skin, it has long been considered as promising route for the administration of drugs, where dermal, regional, or systemic effects are desired. The advantages of the topical route of drug administration include: avoidance of the risks and inconvenience of parenteral treatment; avoidance of the variable absorption and metabolism associated with oral treatment; continuity of drug administration, permitting use of pharmacologically active agents with short biological half-lives and potential reduction of gastrointestinal irritation in systemic administration.Deckner et al,1999
- However, the impermeability of skin is well-known, serving as a barrier to ingress of pathogens and toxic chemicals, and egress of physiologic fluids. This impermeability is the result of normal physiologic changes in developing skin,also results in lesser bioavailability.It is already well known that bioavailability of compounds taken topically example skin whitening ingredients are always lesser than that taken orally or intravenously. Bioavailability of topical dosage forms not intended for absorption has proved to be quite difficult, daunting and extremely challenging.
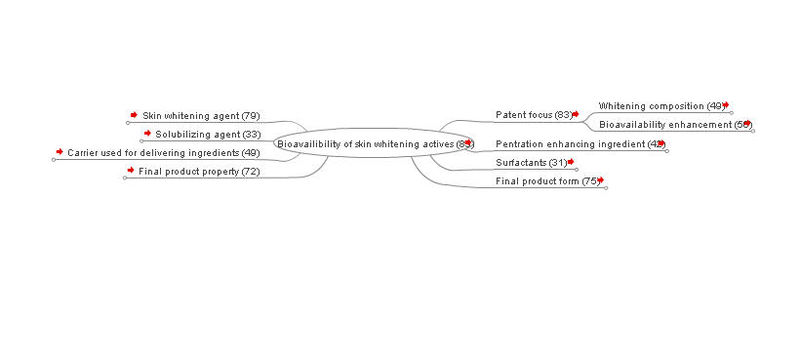
Skin whitening ingredients and enhanced bioavailability
Bioavailability
The determination of the bioavailability of systemically absorbed products is defined as the rate and extent to which the active ingredient or active moiety is absorbed from the drug product and becomes available at the site of action Kanfer and Shargel, 2008. However, when considering products which contain active ingredient(s) not intended for systemic absorption, the US FDA, published the following statement in the US Federal Register (US Federal Register, 2009) where such products“...... may be assessed by (surrogate) measurements intended to reflect the rate and extent to which the active ingredient or moiety becomes available at the site of action”.
Many skin-lightening actives have problems with lesser bioavailability or instability during storage, it also have low affinity to the skin and have little percutaneous absorption. Therefore, several attempts have successfully been made to synthesize conjugates to improve their bioavailabilities. Novel technology has shown great potential for improving the effectiveness and efficiency of delivery of ingredients, e.g.
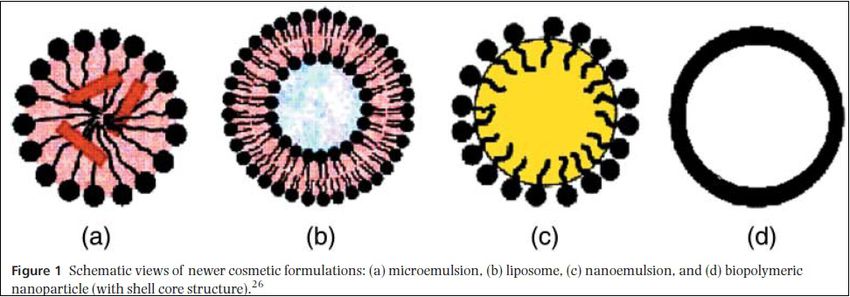
- Microemulsion- An oil-in-water microemulsion have the ability to encapsulate non polar molecules such as lipids, flavorants, antimicrobials, antioxidants, and vitamins, it formulated using lecithin and an alkyl glucoside. It was proposed as a cosmetic vehicle for arbutin and kojic acid, naturally occurring whitening agents. The stability of these compounds are higher in microemulsion than in aqueous solutions.Gallarate et al,2004
- Liposomes- Liposomes encapsulate water and lipid-soluble pharmacologically and cosmetically active components. Liposomes favor the disposition of encapsulated active ingredients in the epidermis and dermis, while the permeation rate is decreased. This helps to fix active ingredients to the outermost skin layers as desired for cosmetic products.Cevec,1997
- Nanoemulsions- Nanoemulsions are emulsions having small droplet size (20–300nm). They could be used for lipophilic as well as hydrophilic substances with enhanced bioavailability
Researcher also used skin penetration enhancer, volatile silicone and several other organic compound which make the ingredients stable and more bioavailable to the skin.
Concept table
Click here to view concept table
Search strategy
- Database: Thomson Innovation
- Coverage:US Grant, GB App, US App, FR App, WO App, DE Util, EP Grant, DE Grant, EP App, DE App, JP Util, JP Grant, JP App, CN Util, CN App, KR Util , KR Grant, KR App, Other, DWPI
- Time Line:1836 - 19 January 2012
| S.No | Concept | Scope | Search strategy | Hits |
| 1 | Class codes of skin whitening AND keywords of bioavailability(English) | Full patent spec. | (424062) OR (424449) OR **** | ### |
| 2 | Class codes of skin whitening AND keywords of bioavailability(German) | Full patent spec. | (A61Q001902) AND (((Bioverfügbarkeit OR **** | ### |
| 3 | Class codes of skin whitening AND keywords of bioavailability(French) | Full patent spec. | (A61Q001902) AND (((biodisponibilité OR **** | ### |
| 4 | F terms of skin whitening AND keywords of bioavailability (English) | full spec in japanese only | (4C083EE16) AND ((Bioavailabilit*3 OR **** | ### |
| 5 | Combined query (Class code of skin whitening and key words of bioavailability) | 1 OR 2 OR 3 or 4 | ### | |
| 6 | highly relevant class codes of bioavailability and keywords of skin whitening | Claims, Title, Abstract | ((514946) OR (514947) OR **** | ### |
| 7 | Class codes of bioavailability AND key words of skin whitening(English) | Claims title abstract | (5140011) OR (5140022) OR **** | ### |
| 8 | english keywords and japanese f terms for bioavailability | Claims title abstract | (4C076FF34) OR (4C076FF03) OR **** | ### |
| 9 | Combined query(Class codes of bio-availability and key words of skin whitening) | 6 OR 7 OR 8 | ### | |
| 10 | Combined query (key word - classcode search) | 5 or 9 | ### | |
| 11 | Skin whitening AND bioavailability key words(English) | Claims, Title, Abstract for skin whitening AND Full patent spec. for bioavailability | (((Skin*1 OR*3 derm*2 OR **** | ### |
| 12 | Class codes of bioavailability AND key words of skin whitening(Geman) | Claims, Title, Abstract for skin whitening AND Full patent spec. for bioavailability | (((Haut OR pelle OR **** | ### |
| 13 | Class codes of bioavailability AND key words of skin whitening(French) | Claims, Title, Abstract for skin whitening AND Full patent spec. for bioavailability | ((((la ADJ2 peau) OR peau OR **** | ### |
| 14 | Combined query (keyword search) | 11 OR 12 OR 13 | ### | |
| 15 | Combined query (Kewyword search and keyword - Classcode search) | 10 OR 14 | ### | |
| 16 | Key words for irrelevant patents | Title, Abstract | (Teeth OR tooth OR**** | ### |
| 17 | Final query | 15 NOT 16 | ### | |
| 18 | L’Oreal | Assignee Applicant, Assignee/Applicant Standardized, Assignee/ applicant original, Assignee Applicant DWPI and US reassignment | "La Institut Lancome" OR **** | ### |
| 19 | L’Oreal Restricted | 17 AND 18 | ### | |
| 20 | Shiseido | Assignee Applicant, Assignee/Applicant Standardized, Assignee/ applicant original, Assignee Applicant DWPI and US reassignment | "SHISEIDO INTERNATIONAL FRANCE" OR **** | ### |
| 21 | Shiseido Restricted | 17 AND 20 | ### | |
| 22 | Kao and Kanebo | Assignee Applicant, Assignee/Applicant Standardized, Assignee/ applicant original, Assignee Applicant DWPI and US reassignment | "LABTEC GESELLSCAFT FA?R TECHNOLOGISCHE FORSCHUNG UND ENTTWICKLUNG MBH" OR**** | ### |
| 23 | Kao and kanebo restricted | 17 AND 22 | ### |
Relevant class codes and definitions
IPC/ECLA classes
| A61 | Medical or Veterinary science; Hygiene | Column1 | Column2 |
| A61Q | Use of cosmetics or similar toilet preparation | ||
| A61Q 19/02 | Preparations for care of the skin =>for chemically bleaching or whitening the skin | ||
| **** | **** | **** |
US Classes
| 424 | Drug, bio-affecting and body treating compositions | |
| 424/62 | Bleach for live hair or skin (e.g., peroxides, etc.) | |
| 424/78.03 | Topical body preparation containing solid synthetic organic polymer as designated organic active ingredient (doai) => Skin cosmetic coating | |
| 424/449 | Transdermal or percutaneous | |
| 514 | Drug, bio-affecting and body treating compositions | |
| 514/1.1 | Designated organic active ingredient containing => Peptide (e.g., protein, etc.) containing DOAI | |
| 514/2.2 | Designated organic active ingredient containing => Peptide (e.g., protein, etc.) containing DOAI=> Bactericidal/permeability-increasing (BPI) protein affecting or utilizing | |
| 514/844 | Cosmetic, facial | |
| 514/946 | Penetrant or absorbent (enhances penetration into subject treated) | |
| 514/947 | Penetrant or absorbent (enhances penetration into subject treated) => Topical application | |
F-Term
| Theme | Title of the theme | F-term | F-term description |
| 4C083 | Cosmetics | 4C083EE16 | Whitening (Tyrosinase inhibition, melanin inhibition) |
| 4C076 | Medicinal preparation | 4C076FF34 | Absorption accelerators or penetrants |
| 4C076FF03 | with absorption, adsorption, or impregnation properties | ||
| 4C076FF32 | Biologically absorbed substances | ||
| 4C086 | Pharmaceuticals containing other organic and inorganic compounds | 4C086NA11 | Absorption improvement |
Taxonomy
Relevant patents
Click here to view Relevant patents
Analysis
Click here to download sample analysis sheet
Trends and Insights
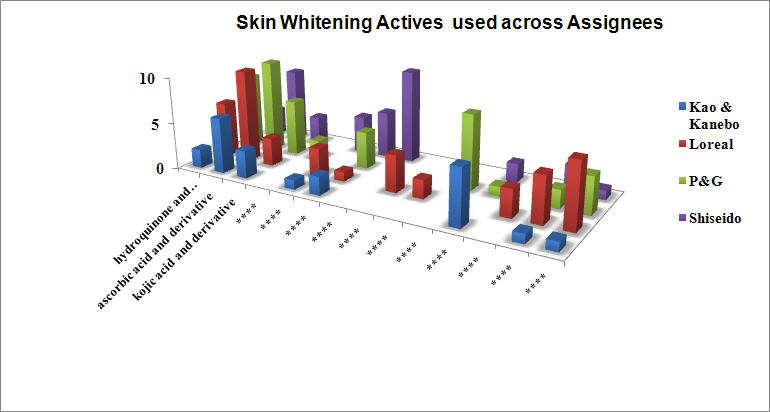
Assigneewise preferred skin whitening actives
Key Points
- Tranexamic acid and derivatives are restricted to Shisheido
- Alpha Hydroxy acid and Tocopherol derivatives are restricted to Loreal
- Vitamin B3 and derivatives are exclusive to P&G
- Loreal exclusively exploits biomolecules such as siRNA and Enzymes in whitening compositions
- P & G uses a novel sodium metabisulfite as skin whitening agent
- Kao and Kanebo prefers natural extract as whitening actives
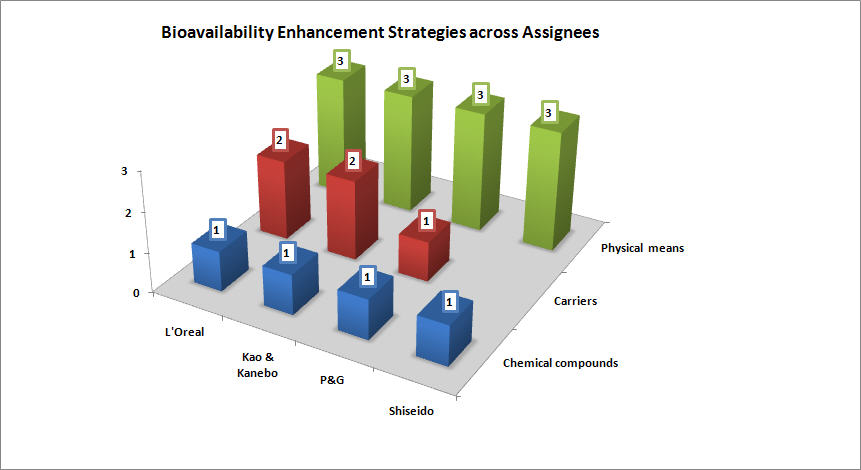
Assignee-wise adopted bioavailability enhancing strategies
Key Points
- Shiseido prefers chemical compounds as penetration enhancers such as silicone compounds, carboxylic acids, etc.
- L’Oreal’s penetration enhancers are mostly carriers such as liposomes, nanoemulsions, etc. followed by physical methods like iontophoresis, ultrasonic waves etc.
- P & G has a preference for carriers followed by physical methods and chemical compounds
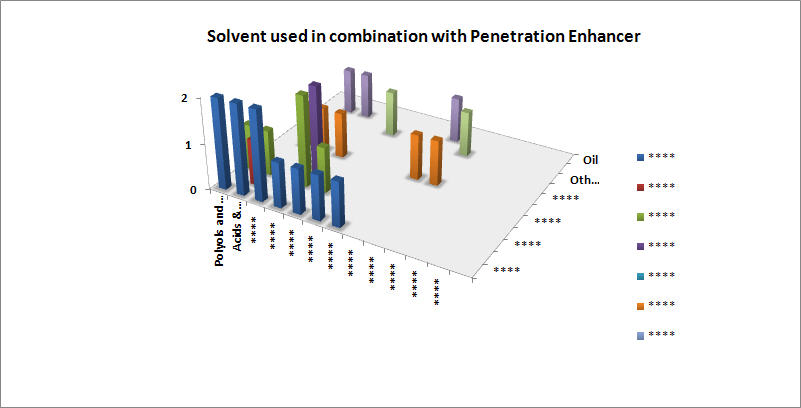
Preferred solvent with respect to penetration enhancer
Key Points
- Vitamins and Salicylic acid derivatives are preferably dissolved in lower alcohols
- Inorganic compounds are used with a wide range of solvents like higher & lower alcohols, acids & derivatives, etc.
- Polyols are used in conjunction with solvents such as glycol, glycerol, lower alcohols and acids
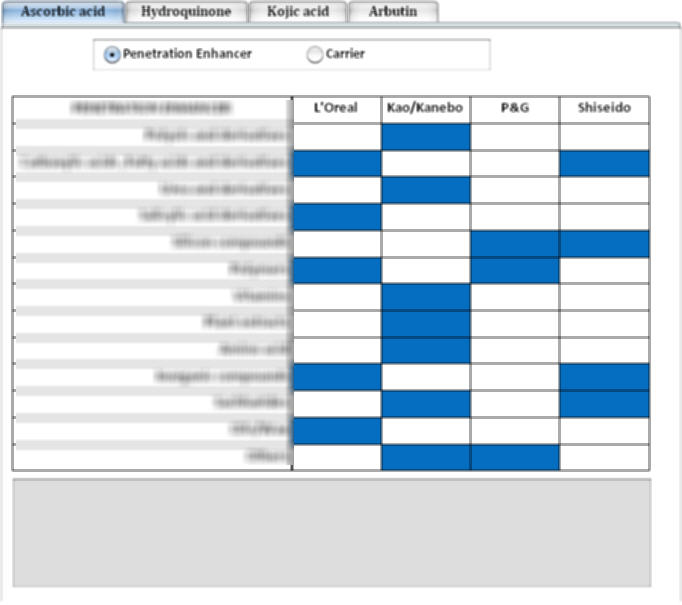
Delivery strategies for whitening actives
- The most common skin-whitening agents have been mapped with the usage of penetration enhancer and carrier across the assignees.
- Click on the appropriate tab for each active.
- Each colored cells will give the corresponding information of delivery ingredients/methods
Dashboard
A preview of the dashboard is shown below.