Transplant Diagnostics (HLA) Market Landscape
Contents
[hide]Introduction
Human leukocyte antigens (HLA) are molecules found on cell surfaces that regulate how the body recognizes and thus possibly rejects foreign tissue transplants. Molecular profiling of HLA sequences, or tissue typing is performed prior to organ or bone marrow transplantations. HLA testing is a key component in determining the compatibility between potential donors and recipients prior to transplantation to maximize the chances of graft survival and minimize serious immunologic transplant complications. The better the match of HLA types, the higher the likelihood of a successful transplant.
- The HLA molecules control the immune response through recognition of 'self' and 'non-self' and the main function of the HLA molecules is presenting the antigen to the T Lymphocytes and initiating the specific immune response
- HLA system constitutes an immunological barrier which must be avoided or otherwise overcome in clinical transplantation
- For Haemopoietic Stem Cell (HSC) transplants, the degree of HLA matching is critical in determining the probability of Graft-versus-Host disease (GvHD)
- In an attempt to minimise these alloresponses, the HLA class I and class II types of the donor and recipient are matched as closely as possible
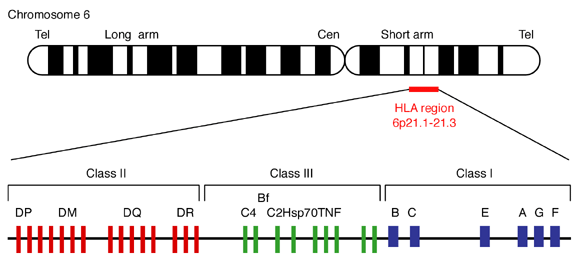
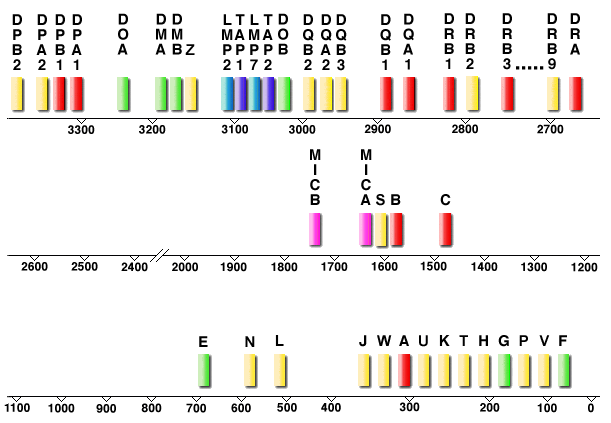
The HLA is located on chromosome 6p21.31 and covers a region of about 3.6 Mbp depending on the haplotype. The classical HLA antigens encoded in each region are HLAA, -B, and -Cw in the class I region, and HLA-DR, -DQ and -DP in the class II region. All class I genes are between 3 and 6 kb, whereas class II genes are 4-11 kb long.
One of the main characteristics of the HLA is its extreme polymorphism. Among the expressed loci, the HLA has the greatest degree of polymorphism in the human genome.
HLA Typing Methods
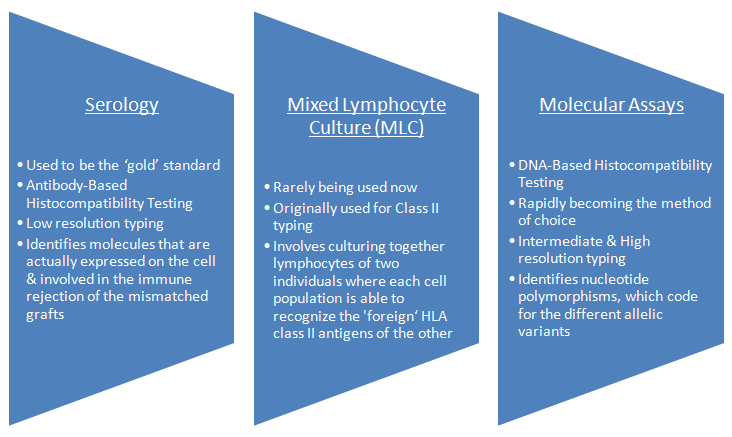
Histocompatibility Testing can be performed using various assays.
- HLA typing by serology is the most common method in routine clinical setting
- Due to rapid advances in technology, molecular assays are now regarded as the 'Method of Choice'
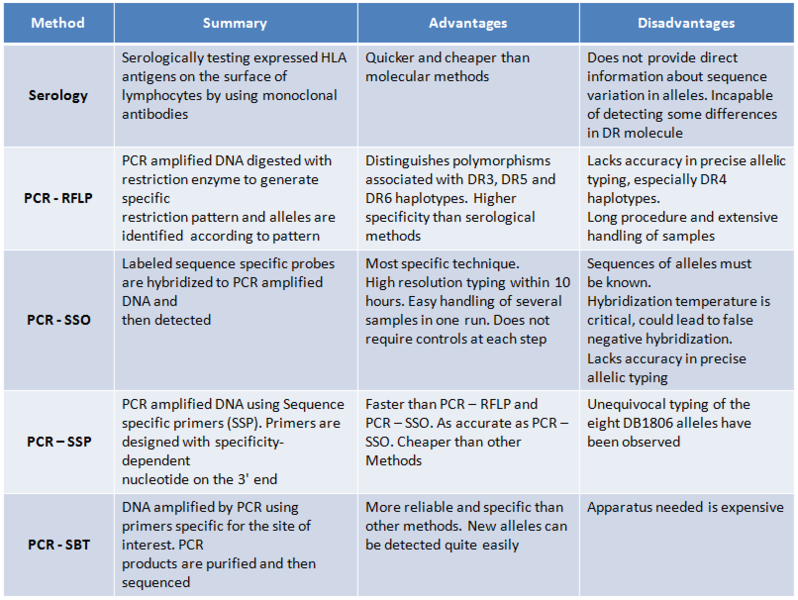
Comparison of HLA Typing Techniques
Market Size
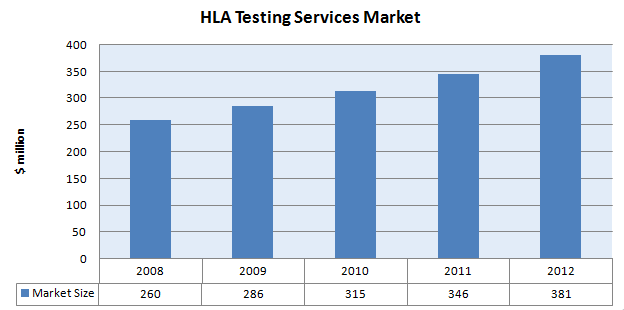
- The market for HLA Testing Services was estimated to be $ 260 million in 2008
- The indicative annual market growth rate is 10% for this space.
Major Players
One Lambda Profile
Company Overview
One Lambda, Inc. is a medical diagnostic company based in Canoga Park, California. It was founded by Paul Terasaki and George Ayoub in April, 1984.
One Lambda develops and distributes histocompatibility reagents and semi-automated laboratory equipment for hospitals, research centers, and institutions involved in human organ transplantation and paternity testing. The reagents produced are primarily used to determine genetic compatibility between an organ donor and a recipient. Using the Terasaki tray design, Human Leukocyte Antigen (HLA) typing tests or histocompatibility tests are produced with these reagents.
The company markets several lines of HLA typing tests utilizing both serological and molecular technologies. One Lambda also manufactures a comprehensive line of laboratory instrumentation and computer software products that are used to simplify and automate testing procedures and final test evaluation.
One Lambda presently services more than 1,400 laboratories worldwide.
Key Facts
| Website | www.onelambda.com |
| Administrative Department | 21200 Oxnard Street Woodland Hills, CA 91367, USA |
| R&D and Manufacturing | 21001 Kittridge Street Canoga Park, CA 91303, USA Tel: +1 818 702 0042 Fax: +1 818 702 6904 |
| Regional Office | Synergie - Le Millenaire 770, Rue Alfred Nobel 34000 Montpellier, France Tel: +33 4 99 13 60 20 Fax: +33 4 67 64 60 40 |
| Ownership | Private |
| Fiscal Year Ending | 31, December |
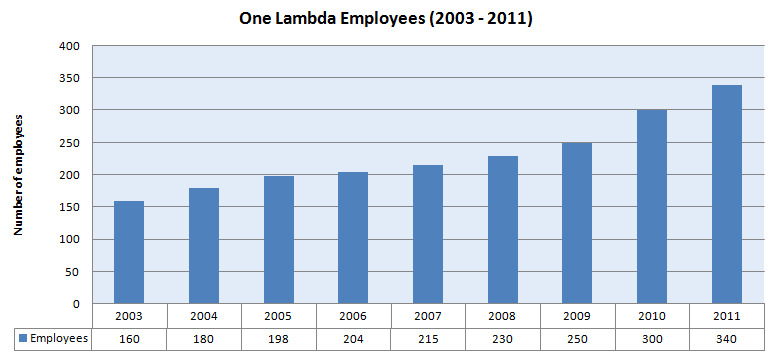
| Employees (2011) | 340 |
| Revenues (2010) | $ 87 M |
Financials
- One Lambda has recorded revenues of $ 87 Million in 2010 according to Privco, a private company information database. According to Experian credit report it was $ 87.5 Million in 2010.
- CAGR of revenues for the period 2004 - 2010 is 7.62%
Human Resource Metrics
- Total number of employees in One Lambda at present (2011) are about 340
- CAGR of employees for the period 2003 - 2011 is 9.88%
- CAGR of employees for the period 2004 - 2010 is 8.89%
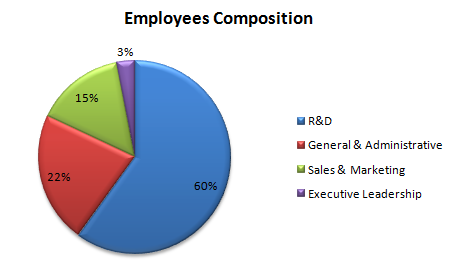
Function-wise Data
| Function | Growth Rate (Nov 2010 - Oct 2011) |
| R&D | 29% |
| General & Administrative | 0% |
| Sales & Marketing | -17% |
| Executive Leadership | 0% |
Funding Details
| Date | Investor | Value ($) | Funding Type |
| 2005 | National Institute of Health | 885,379 | Grant |
| 2006 | National Institute of Health | 836,774 | Grant |
| 2007 | National Institute of Health | 779,516 | Grant |
Source: Privco Report, News Articles
Recent News and Developments
| S.No. | Date | Details |
| 1 | October 2011 | One Lambda, Inc. won a $1.5 million federal contract from the U.S. Naval Medical Logistics Command, Fort Detrick, Md., to develop the next generation high resolution human leukocyte antigen (HLA) typing reagents for the NMRC HLA Typing Program and Bone Marrow Research Directorate |
| 2 | April 2011 | One Lambda, Inc. and the National Kidney Registry announced the introduction of The Toolbox, a new innovative Web module. The Toolbox is designed to help medical professionals make intelligent decisions on donor selection based on antibody profiles of patients |
| 3 | December 2010 | Luminex Corporation and One Lambda, Inc. have agreed to a long-term renewal of their 10-year strategic partnership aimed at developing platforms and technologies for human leukocyte antigen (HLA) typing and antibody screening for the organ transplantation market |
Source: News Articles