Introduction
- RNA interference (RNAi), is a technique in which exogenous, double-stranded RNAs (dsRNAs) that are complimentary to known mRNAs, are introduced into a cell to specifically destroy that particular mRNA, thereby diminishing or abolishing gene expression.
- This technology considerably bolsters functional genomics to aid in the identification of novel genes involved in disease processes and thus can be used for medicament and for delivery as therapeutics. Source
- RNA interference was known by other names, including post transcriptional gene silencing and quelling. Source
Effector RNA molecules
RNAi pathways are guided by small RNAs that include
SiRNA:
- Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of 20-25 nucleotide-long double-stranded RNA molecules.
- SiRNAs can also be exogenously (artificially) introduced into cells by various transfection methods to bring about the specific knockdown of a gene of interest. Source
miRNA:
- microRNAs (miRNA) are single-stranded RNA molecules of about 21–23 nucleotides in length, which regulate gene expression.
- miRNAs are encoded by genes from whose DNA they are transcribed but miRNAs are not translated into protein (non-coding RNA); instead each primary transcript (a pri-miRNA) is processed into a short stem-loop structure called a pre-miRNA and finally into a functional miRNA.
- Mature miRNA molecules are partially complementary to one or more messenger RNA (mRNA) molecules, and their main function is to down-regulate gene expression. Source
shRNA:
- A small hairpin RNA or short hairpin RNA (shRNA) is a sequence of RNA that makes a tight hairpin turn that can be used to silence gene expression via RNA interference.
shRNA uses a vector introduced into cells and utilizes the U6 promoter to ensure that the shRNA is always expressed. This vector is usually passed on to daughter cells, allowing the gene silencing to be inherited. The shRNA hairpin structure is cleaved by the cellular machinery into siRNA, which is then bound to the RNA-induced silencing complex (RISC). This complex binds to and cleaves mRNAs which match the siRNA that is bound to it. Source
Others:
- In addition to miRNAs and siRNAs, other innate RNAi effectors have been identified.
- One class of these is the Piwi-interacting RNAs (piRNAs). piRNAs seem to be uniquely expressed in the mammalian germline, particularly in the testes. The functional role of piRNAs is currently unclear, but a role in spermatogenesis is likely.
- A number of other small RNAs associated with RNAi have been identified in different species, including trans-activating siRNAs (tasiRNAs), studied in plants and nematodes, and small scan RNAs (ScnRNAs), found in Tetrahymena. Source
Cellular Mechanism
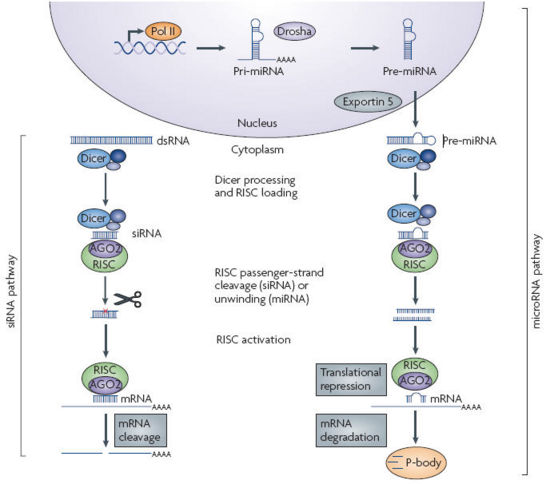
- RNAi is an RNA-dependent gene silencing process that is mediated by the RNA-induced silencing complex (RISC) and is initiated by short double-stranded RNA molecules in the cytoplasm, where they interact with the catalytic RISC component argonaute.
- When the dsRNA is exogenous, coming from infection by a virus with an RNA genome or laboratory manipulations, the RNA is imported directly into the cytoplasm and cleaved to short fragments by the enzyme dicer.
- The initiating dsRNA can also be endogenous, as in pre-microRNAs expressed from RNA-coding genes in the genome. The primary transcripts from such genes are first processed to the characteristic stem-loop structure of pre-miRNA in the nucleus, then exported to the cytoplasm to be cleaved by dicer. Thus the two pathways for exogenous and endogenous dsRNA converge at the RISC complex, which mediates gene silencing effects. Source
siRNA Delivery
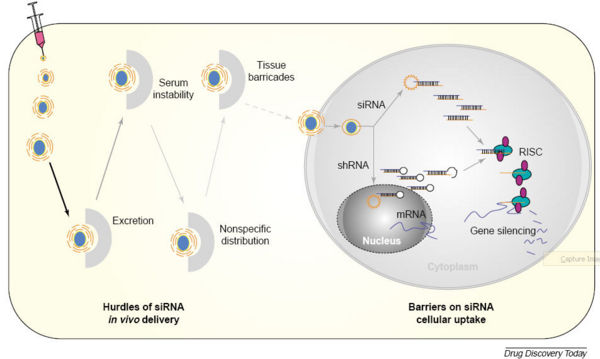
- Delivery of siRNA to tissue is a problem both because the material must reach the target organ and must also enter the cytoplasm of target cells.
- Naked RNA cannot penetrate cellular membranes, so systemic delivery of naked siRNA is unlikely to be successful. Naked RNA is quickly degraded by RNAse activity in serum and even siRNA chemically modified to be more stable has a half-life of only a few hours at most.
- For these reasons, other mechanisms to deliver siRNA to target cells has been devised. Source
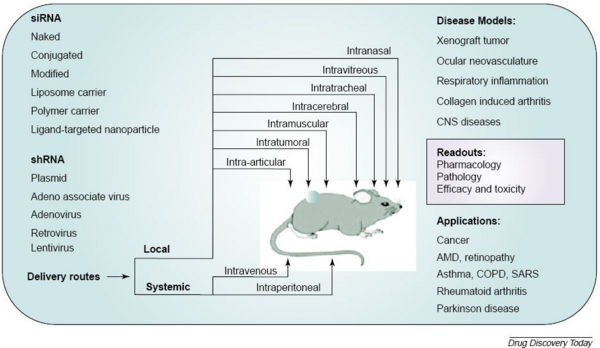
Other mechanisms of siRNA delivery include:
- Viral delivery including Adenoviral, Lentiviral, Baculoviral, Adeno-associated viral vectors etc.
- Bacterial delivery
- Plasmid delivery
- Peptides and polymers
- Liposomes and lipoplexes
- Nano particles
- Antibodies
Applications
• RNAi technology is proving to be useful to analyze quickly the functions of a number of genes in a wide variety of organisms.
• RNAi technology has been successfully applied to identify genes with essential roles in biochemical signaling cascades, embryonic development, and other basic cellular process.
• In plants, gene knockdown-related functional studies are being carried out efficiently when transgenes are present in the form of hairpin (or RNAi) constructs. Plant endotoxins could also be removed if the toxin biosynthesis genes are targeted with the RNAi constructs, like theobromine synthase of the coffee plant was knocked down with the hairpin construct of the transgene, leading to the production of decaffeinated coffee plants.
• RNAi may facilitate drug screening and development by identifying genes that can confer drug resistance or genes whose mutant phenotypes are ameliorated by drug treatment, providing information about the modes of action of novel compounds.
• It may also be a method of choice to study the simultaneous functions of a number of analogous genes in organisms in which redundancy exists with respect to a particular function, because many of these genes can be silenced simultaneously.
• siRNAs have been shown to inhibit infection by human immunodeficiency virus, poliovirus, and hepatitis C virus in cultured cell lines. siRNAs can successfully be used to silence genes expressed in respiratory syncytial virus, an RNA virus that causes severe respiratory disease in neonates and infants. siRNA treatment has also been shown to reduce the expression of the BCR-ABL oncoprotein in leukemia and lymphoma cell lines, leading to apoptosis in these cells. With respect to future medical applications, siRNA-based therapy seems to have a great potential to combat carcinomas, myeloma, and cancer caused by overexpression of an oncoprotein or generation of an oncoprotein by chromosomal translocation and point mutations. Source
Intellectual Property
Search Strategy
Micropat
Years: 1836 to 2008
| S.No | Concept | Scope | Query | # of hits |
| 1 | siRNA | Claims, Title or Abstract | (siRNA*1 OR (short ADJ1 interfering ADJ1 RNA*1) OR (short ADJ1 interfering ADJ1 nucleic ADJ1 acid*1) OR (short ADJ1 interfering ADJ1 NA*1) OR siNA OR siNAs OR (si ADJ1 RNA*1)) | 7203 |
| 2 | RNAi | Claims, Title or Abstract | (RNA*1 NEAR2 interfer*) OR RNAi* | 9057 |
| 3 | 1 OR 2 | 13541 | ||
| 4 | Delivery | Claims, Title or Abstract | (Deliver* OR Formulation*1 OR conjugat*1 OR administ*) | 1418602 |
| 5 | 3 AND 4 | 5964 | ||
| 6 | Unique records | 3592 |
Non-Patents
Scirus and PubMed
| S.No | Query | # of hits |
| 1 | (((siRNA OR (short interfering RNA) OR (short interfering nucleic acid) OR siNA OR siNAs) OR (RNA interfer) OR RNAi OR (Interfering RNA)) AND (Deliver OR Delivery OR Formulation OR conjugat OR administ)) | ~2000 articles (399 reviews) |
Note: The search strategy can be modified in accordance to the Clients' requirements.
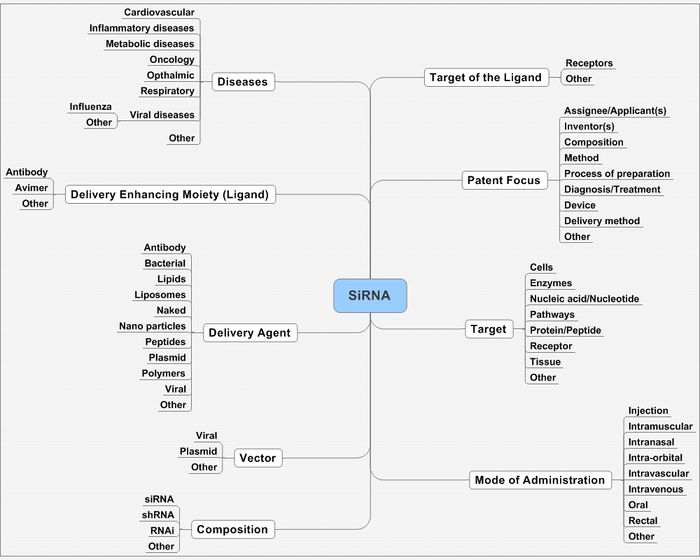
It can be narrowed down at concepts like
- Delivery mechanism
- Mode of administration
- Target
- Diseases
- Composition, etc.
Analysis Taxonomy
Analysis
Patent Literature
| S.No | Patent / Publication Number | Date of Publication | Application Date | Assignee / Applicant | Title | Dolcera Summary |
| 1 | EP1946761 | 7/23/2008 | 10/17/2006 | Otsuka Pharmaceutical Co., Ltd. | CARRIER COMPOSITION FOR NUCLEIC ACID TRANSPORT | A nucleic acid like siRNA carrier composition comprising of cationic lipids is used in the siRNA delivery to the target cells with low toxicity and high safety. |
| 2 | US7345027 | 3/18/2008 | 6/8/2006 | The Trustees of the University of Pennsylvania | Compositions and methods for siRNA inhibition of angiogenesis | A method of inhibiting angiogenesis and its related disorders using RNA interference, where siRNA alone or in combination with a pharmaceutical agent delivered using liposomes, specific to vascular endothelial growth factor (VEGF) gene and the VEGF receptor genes Flt-1 and Flk-1/KDR, inhibit expression of these genes. |
| 3 | US20040242518 | 12/2/2004 | 9/29/2003 | Massachusetts Institute of Technology | Influenza therapeutic | A method of inhibiting influenza infection or replication using RNAi technology, where siRNA is targeted to the infected cells containing the viral RNA using a delivery agent like polymers, peptides, liposomes, etc. |
| 4 | US20070003519 | 1/4/2007 | 10/20/2006 | None | Targets for tumor growth inhibition | A method of treating cancer using RNAi technology, where siRNA interferes with target ICT1031, ICT1024, ICT 1025, or ICT1003 gene expression and causes post-transcriptional silencing, where siRNA is delivered in the naked or vector form. |
| 5 | US20080020058 | 1/24/2008 | 10/24/2006 | Sirna Therapeutics, Inc. | Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules | A method of treating diseases associated with gene expression using siRNA along with delivery agents like nano-particles. |
| 6 | WO2006086772 | 8/17/2006 | 2/10/2006 | GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES | METHOD OF DIAGNOSING AND TREATING CANCER USING B-CATENIN SPLICE VARIANTS | A method and composition for treating f3-catenin gene (CTNNB 1) related diseases like cancer, using RNAi construct like siRNA, delivered using liposomes. The siRNA attenuates expression of a gene resulting in reducing proliferation. |
| 7 | WO2008054544 | 5/8/2008 | 5/22/2007 | THE CBR INSTITUTE FOR BIOMEDICAL RESEARCH, INC | NARASIMHASWAMY, Manjunath | SHANKAR, Premlata | KUMAR, Priti | METHOD FOR DELIVERY ACROSS THE BLOOD BRAIN BARRIER | A composition and method of delivering therapeutic agents like siRNA, shRNAs across the blood-brain barrier to the target cells or tissues for the treatment of neurologically related disorders. |
| 8 | WO2008039254 | 4/3/2008 | 5/31/2007 | GOVERNMENT OF THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES | YINGLING, Yaroslava, G. | SHAPIRO, Bruce, A. | RNA NANOPARTICLES AND NANOTUBES | A method of providing polyvalent RNA nanoparticles with RNA motifs as building blocks to form RNA nanotubes which are further suitable for therapeutic or diagnostic use in a number of diseases or disorders. |