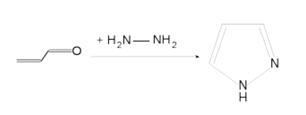Alopecia - Hair Loss
Rationale
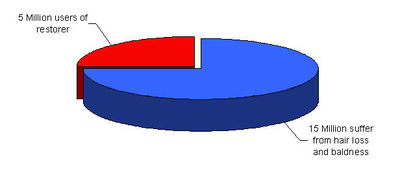
- "Medication for men plagued by hair loss has become a topic of interest in Japan since a drug company began marketing it at the end of last year." March 5th, 2006 – [1]
- "An increasing number of companies are apparently turning the Chinese fear of a bald spot into big bucks with some doing so well they are branching out into other countries." February 16, 2006 – [2]
- "There is something in the air, or should we say in the hair, these days. Scientific research into hair loss remedies has never been more active or more exciting." June 7, 2006 - [3]
Alopecia IPMap
Introduction
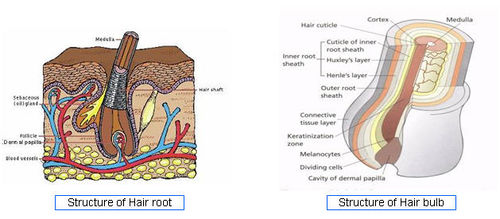
Hair basics
- Hair is a complex and delicate part of the body.
- Keeping it healthy and beautiful is a challenge.
- Hair grows everywhere on the body with the exception of the lips, eyelids, the palms of the hands and soles of the feet.
- Hair is basically a form of skin.
- Hair is made up of a protein called keratin.
- Each shaft of hair is made of two or three inter-twined layers of keratin which grow from a follicle beneath the skin.
- Hair Structure - [4]
- Hair Cycle - [5]
What causes hair loss?
- Decreased growth of the hair
- Increased shedding of the hair
- Breakage of hairs
- Conversion of thick terminal hairs to thin vellus hairs
Both men and women lose hair for similar reasons. Hair loss in men is often more dramatic, and follows a specific pattern of loss, one of which has been termed “Male Pattern Baldness" or "Androgenetic Alopecia".
Type of alopecia
- Alopecia Areata (AA): Used to describe hair loss occurring in patches anywhere on the body.
- Alopecia Totalis (AT): Total loss of the hair on the scalp.
- Alopecia Universalis (AU): Total loss of all hair on the body.
- Alopecia Barbae: Loss of facial hair (for a man) especially in the beard area.
- Alopecia Mucinosa: A type of alopecia which results in scaley patches.
- Androgenetic Alopecia (AGA): Also known as male pattern baldness. It is a thinning of the hair to an almost transparent state, in both men or women. It is thought to be a hereditary form of hair loss.
- Traction Alopecia: Traction alopecia is usually due to excessive pulling or tension on hair shafts as a result of certain hair styles. It is seen more often in women, particularly those of East Indian and Afro-Caribbean origin. Hair loss depends on the way the hair is being pulled. Prolonged traction alopecia can stop new hair follicles developing and lead to permanent hair loss.
- Anagen Effluvium: This hair loss is generally caused by chemicals such as those used to treat cancer. Initially it causes patchy hair loss, which often then becomes total hair loss. The good news is that when you stop using these chemicals the hair normally grows back (usually about 6 months later). Other drugs also can cause hair loss. Many medicines used to treat even common diseases can cause hair loss.
- Scarring Alopecia: A form of alopecia which leaves scarring on the area of hair loss.
- Telogen Effluvium: A form of hair loss where more than normal amounts of hair fall out. There is a general 'thinning' of the hair. Unlike some other hair and scalp conditions, it is temporary and the hair growth usually recovers. source
Androgenetic alopecia
- Gradual Onset.
- Transition from large, thick, pigmented terminal hairs to thinner, shorter, indeterminate hairs and finally to short, wispy, nonpigmented vellus hairs in the involved areas.
- Characterised by a receding hairline and/or hair loss on the top of the head.
Main causes
- Genetic predisposition
- Hormonal effect of androgen
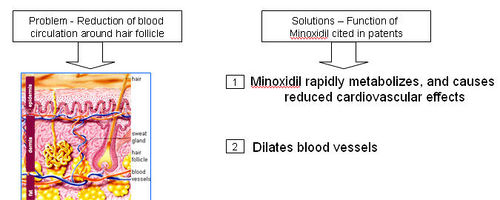
- Reduction of blood circulation around hair follicle
- Deactivation of hair matrix cells
Some facts from Japan
- Market size: ¥ 30 Billion
- Number of products: more than 100
(JICST-EPlus - Japanese Science & Technology)
Goals
The goal of this report is to:
- Summarize IP activity over the years
- Identify major players
- Conduct patent analysis
a) Composition b) Nature c) Action
- Technology mapping based on patent anlysis elements
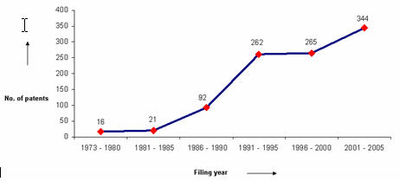
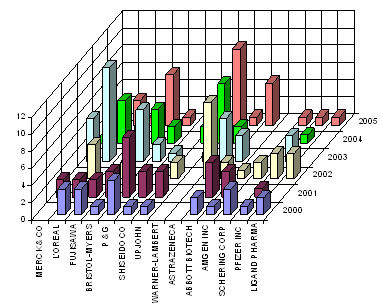
IP activity over years
The graph indicates:
- Number of patents filed every 5 years (except for first 7 years).
- First solution proposed in 1973
- Filing trend indicates steep rise in activity recently.
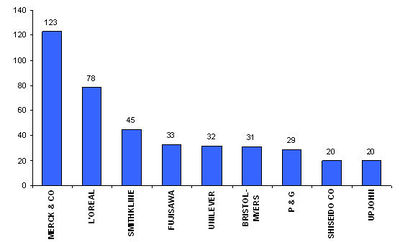
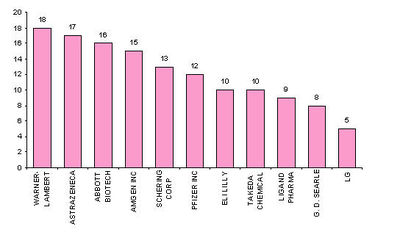
Major players
- Active assignees
Assignees currently active with more than 5 patents to their credit during 2000-2005.
- Warner with 9 patents,
- Bristol with 6 and
- Abbott with 5.
Approach and Methodalogy
- A broad search was conducted on hair loss patents.
- Patent information was sourced through SIP.
- A set of patents was selected for analysis.
Treatment Approaches
Composition of treatment for causes are identified and categorized as follows:
- Anti-androgens
- Vasodilators (Minoxidil)
- Double action (Anti-androgen + Vasodilator)
- Hair matrix cells activator
| Treatment Approaches | Pathway effected |
| Anti-androgens | Testosterone pathway |
| Vasodilators (eg. Minoxidil) | NO/ cGMP Pathway |
| Hair matrix cells activator |
|
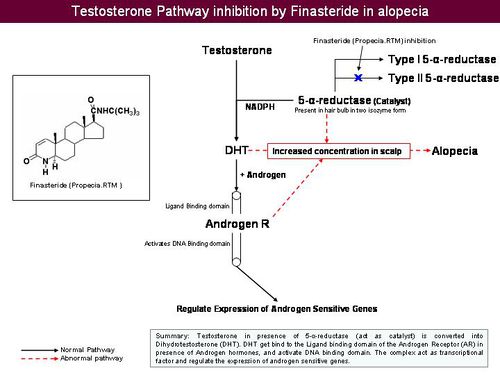
Anti-androgens
- Anti-androgens are used in hormone therapy.
- Anti-androgens are designed to affect the hormones made in the adrenal glands. They don't stop the hormones from being made, but they stop them from having an effect leading to hair loss.
What causes hair loss?
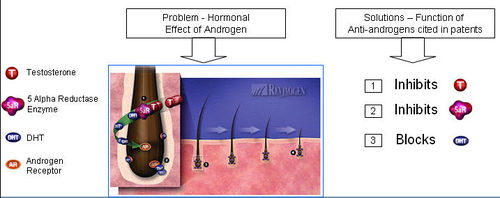
- Testosterone is reduced to its active metabolite, Dihydrotestosterone (DHT) by the enzyme 5 alpha reductase.
- DHT attaches to androgen receptor sites at the hair follicle.
- DHT causes gradual miniaturization of the follicle, which eventually results in hair loss.
How do anti-androgens treat hair loss?
- Anti-androgens compete with DHT to bind to the androgen receptor.
- Upon binding of anti-androgen in place of DHT, follicle miniaturization is lowered and hair loss prevented.
Functions of Anti-androgen
IP Map for anti-androgen
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20060009430
BLOTECH (2004) |
Natural extracts | Palmetto berry extract (fatty acids & sterols), Pumpkin seed extract (Vitamins-B, alpha-linolenic acid, amino acids and phytosterols), Quercetin (Flavonoids) and Beta-sitosterol (Rice bran, wheat germ, corn oils and soybeans) | Fatty acids – Inhibit testosterone
Sterols - Mechanism of action unknown. Quercetin results in cell growth cycle. Beta-sitosterol reduce inflammation on scalp |
| US20060009427
WARNER LAMBERT(2004) |
Organic compound | New class of 4-cycloalkoxy benzonitrile derivatives and salts | Acts as androgen receptor modulators and blocks formation of DHT. |
| US20050085467
WARNER LAMBERT(2004) |
Organic compound | New class of 6-sulfonamido-quinolin-2-one and 6-sulfonamido-2-oxo-chromene derivatives. | The compounds inhibit, or decrease, activation of androgen receptor by androgens. |
| US20050118282
APHIOS Corp (2003) |
Natural extracts | Supercritical fluid isolate of Saw Palmetto and Sperol (Serenoa repens berry) and their analogs or derivatives. | Modulates androgenic activity by inhibiting 5.alpha.-reductase activity. |
| US20060009429
Fundacion Pablo Cassara (2003) |
Nucleotide | Pharmacologically active oligonucleotides (encompass both DNA and S-DNA bond) | Oligonucleotides inhibit androgen receptor (AR) expression at very low concentrations in skin and hair follicle |
| US20030007941
PFIZER INC (2001) |
Organic compound | Thyromimetic compounds (structurally similar to thyronine) with finasteride, or cyproterone acetate | Activates thyroid hormone receptors in hair follicle which in turn promote elasticisation of follicle walls and hair follicle |
| US20030073616
N/A (1995) |
Peptides/nucleic acid | Bradykinin antagonist (peptide of plasma origin from kininogen precursor-kallikrein) | Inhibit synthesis of bradykinin receptors or compounds by binding to B2 receptor |
| EP0279010
KAO Corp (1987) |
Natural extracts | Walnut extract (leaves/pericarps) with an organic solvent | Blocks formation of DHT |
Minoxidil (Vasodilators)
- Minoxidil is a "potassium channel opener" that leads to vasodilation.
- The drug is available in two forms. Oral minoxidil is used to treat high blood pressure and the topical solution form is used to treat hair loss and baldness.
What causes hair loss?
- A thick network of tiny veins and arteries lines the outer wall of the follicle. Blood pumps through the bulb and hair via this network.
- DHT accumulates in the hair follicles and roots, constricting the blood supply of oxygen and nutrients to the hair roots; which is also seen to possibly contribute towards hair loss.
How does Minoxidal treat hair loss?
- Minoxidal is applied to the scalp topically, where it dilates blood vessels in the scalp and sustains the hair follicles for longer period of time.
- Minoxidil is thought to have a direct mitogenic effect on epidermal cells, as has been observed both in vitro in vivo. Though the mechanism of its action for causing cell proliferation is not very clear, minoxidil is thought to prevent intracellular calcium entry. Calcium normally enhances epidermal growth factors to inhibit hair growth, and Minoxidil by getting converted to minoxidil sulfate acts as a potassium channel agonist and enhances potassium ion permeability to prevent calcium ions from entering into cells.source
- Minoxidil sulfate (MS) appears to be the active metabolite responsible for hair growth stimulation.
Functions of Vasodilators
IP Map for Vasodilators
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20040157856
WARNER LAMBERT(2002) |
Organic compound | Benzopyran compounds | Rapidly metabolizes, and causes reduced cardiovascular effects as compared to other known potassium channel openers |
| US20050053572
LG HOUSEHOLD & HEALTH CARE(2001) |
Natural extracts | Sophora flavescens extract (alkaloids & flavonoids, luteolin-7-glucose and cytosine) Hinokitiol (Taiwan hinoki oil, Aomori, Western Red Cedar oil) and Nicotinamide (Vitamin B complex) | Promotes function of cell activity and dilates blood vessels |
Double action (Anti-androgen + Vasodilator)
- Combination of Vasodilator + Anti-androgen (double action) composition for effective treatment of Male-Pattern Baldness.
What is the problem with using only Anti-androgen therapy?
- Anti-androgen is not effective in addressing the issue of vasocontriction around hair follicles due to sebum oil build up.
- Anti-androgen only prevent binding of DHT to androgen receptors. However, the effects of improper oxygen and nutrient supply to the brain due to vasocontriction still remains and gradually causes hair loss.
What is the problem with using only Vasodilator (or Minoxidil only) therapy?
- Vasodilator or Minoxidil-based products are generally not effective in stopping hair loss as vasodilators (or Minoxidil) does not block the harmful effects of DHT in the scalp and hair follicles.
- Vasodilators or Minoxidil simply dilates blood vessels in the scalp. However, the harmful DHT is still being produced in the body and still getting into the scalp and hair follicles and causing eventual hair loss.
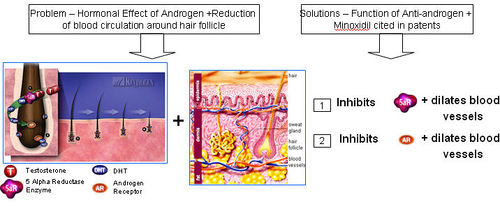
How is the combination of Anti-androgens and Vasodilator (or Minoxidil) effective?
- Anti-androgens target the problem of DHT binding to androgen receptors and prevents follicle miniaturization.
- Vasodilators like Minoxidil causes vasodilation and therefore improves supply of oxygen and nutrients to the hair follicle and roots.
- Combination therapy therefore proves to be much more effective than individual therapy.
==== Functions of (Anti-androgen + Vasodilators) ==== Anti-androgen and Minoxidil
IP Map for (Anti-androgen + Vasodilators)
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20060052405
N/A(2000) |
Peptides | Testosterone blocker or vascular toner (Flutamide, cyproterone acetate, spironolactone, progesterone, or analogs or derivatives) and minoxidil mixed along with non-retinoid penetration enhance and sunscreen | Inhibits 5.alpha.-reductase activity (block DHT) and increase blood flow on the scalp |
| US20050123577
L'OREAL(2000) |
Peptides | Prostaglandin (polyunsaturated fatty acids) EP-2, EP-3 EP-4 receptor agonist with Minoxidil, 2,4-diaminopyrimidine 3-oxide, and Aminexil, cyclic AMP | Minoxidil (designed to mimic nitric oxide's effects) grows hair via prostaglandin-H synthase stimulation. EP-3 and EP-4 are expressed in anagen hair follicles which induce a reduction in the level of cAMP |
| US6447762
COLOMER GROUP(1999) |
Natural extract | Hop extract (oil contains terpenes and humulene), Rosemary extract (hydroalcohol), Swertia extract (glycol with a swertiamarin), Silanodiol salicylate (biologically active silicon compound) | Inhibits activity of 5-alpha-reductase, protects follicular cell membranes by neutralizing action of oxidation reaction in tissues, stimulates hair follicles and blood circulation to the hair root, supplies oxygen and nutrients to base of follicle, retains humidity, avoids dehydration of scalp |
Hair matrix cell activator
Hair matrix cell activator is a substance that acts at the matrix cells in the hair follicle preventing their degradation.
What causes hair loss?
- Stem cells are interspersed within the basal layer of the outer root sheath and in an area called the bulge.
- Stem cells migrate to hair matrix where they start to divide and differentiate, under the influence of substances produced by cells of the dermal papilla.
- Perifollicular matrix cells undergo slow degradation which prevents follicle stimulation.
- Hair follicle activation is required for hair growth and thus inhibition of follicle activation eventually leads to hair loss.
How does hair cell matrix activator treat hair loss?
- Hair cell matrix activator slows down and inhibits degradation of the perifollicular matrix.
- This leads to an increase in hair follicle matrix cells that differentiate from progenitor stem cells.
- Matrix activator allows activation of hair matrix cells and therefore follicle stimulation leading to hair growth.
==== Functions of Hair matrix cell activator ==== Hair matrix cell activator
IP Map for Hair matrix cell activator
| Pat/Pub# | Nature | Composition | Composition action |
|---|---|---|---|
| US20020052498
SHISEIDO(1999) |
Organic compound | (2-substituted oxyphenyl) alkanamide derivative and its salt | Mechanism of action has not been made clear, having excellent hair follicle activating action and regrowth promoting effect |
| US20040071647
L'OREAL(1998) |
Peptides | Metalloprotease (MMP-9) inhibitor (thiol or a hydroxamate) other than chelating calcium ions | Reducing the expression of MMPs (Metalloproteases) in the scalp - slow down or inhibit the degradation of the perifollicular matrix (extracellular matrix surround the hair follicle) |
Technology mapping based on patents anlyzed
IPMap: Composition nature matrix
| Year | Organic Compound | Natural extracts | Peptides | Nucleotides | Natural extract + Organic comp |
|---|---|---|---|---|---|
| 2005 | .... | .... | .... | .... | UNILEVER (1) |
| 2004 | WARNER (1) | BLOTECH (1) | .... | .... | KAO (1) |
| 2003 | WARNER (1) | APHIOS (1) | .... | FUNDACION (1) | .... |
| 2002 | WARNER (1) | .... | .... | .... | .... |
| 2001 | PFIZER (1) | LG HEALTH-CARE (1) | .... | .... | .... |
| 2000 | .... | .... | L’OREAL (1) / N/A (1) | .... | .... |
| 1999 | SHISEDIO (1) | COLOMER (1) | .... | .... | .... |
| 1998 | .... | .... | L’OREAL (1) | .... | .... |
| 1995 | .... | .... | N/A (1) | .... | .... |
| 1987 | .... | KAO (1) | .... | .... | .... |
| 1982 | UNILEVER (1) | .... | .... | .... | .... |
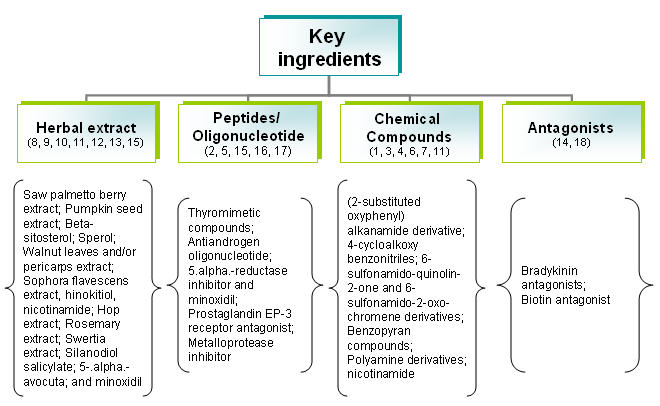
Focus of patents
| Focus of patents | Patent no. | Rec. no. |
|---|---|---|
| 2-substituted oxyphenyl alkanamide derivative having excellent hair growth effect. | US20020052498 | 1 |
| Thyromimetic compounds, and its role in treating hair loss | US20030007941 | 2 |
| Saw Palmetto berry extract, pumpkin seed extract, sitosterol and quercetin for the treatment and prevention of the biologically detrimental effects of DHT | US20060009430 | 3 |
| 4-cycloalkoxy benzonitriles and its use as androgen receptor modulators | US20060009427 | 4 |
| Supercritical fluid isolate of Saw Palmetto, Sperol for inhibition of 5-.alpha.-reductase activity | US20050118282 | 5 |
| New class of quinolin-2-ones and chromen-2-ones andtheir use as androgen receptor antagonists | US20050085467 | 6 |
| Antiandrogen oligonucleotides usable for the treatment of dermatological androgen-related disorders | US20060009429 | 7 |
| Bradykinin antagonists for stimulating or inducing hair growth and/or arresting hair loss | US20030073616 | 8 |
| Extract from walnut leaves and/or pericarps as 5 alpha -reductase inhibitor | EP0279010 | 9 |
| Stimulating hair growth using benzopyrans | US20040157856 | 10 |
| Sophora flavescens extract, Coicis semen extract, clove extract, etc for promoting hair growth, function of cell activity and dilating peripheral blood vessels. | US20050053572 | 11 |
| Compositions to prevent or reduce hair loss | US20060052405 | 12 |
| Prostaglandin EP-3 receptor antagonists for reducing hair loss | US20050123577 | 13 |
| Synergic effect arising from the interaction of active ingredients, consisting of three plant extracts and a synthetic organosilicic compound for prevent hair loss and stimulate hair growth | US6447762 | 14 |
| Metalloprotease inhibitors to induce and/or stimulate the growth | US20040071647 | 15 |
| Method of decreasing sebum production and pore size | US20050277699 | 16 |
| Method for reducing sebum on the hair and skin | US4529587 | 17 |
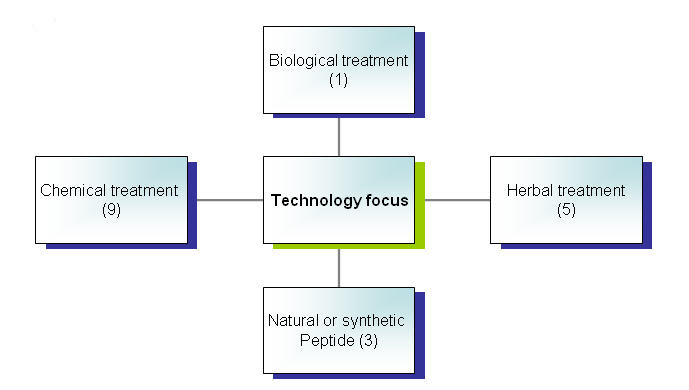
Technology focus
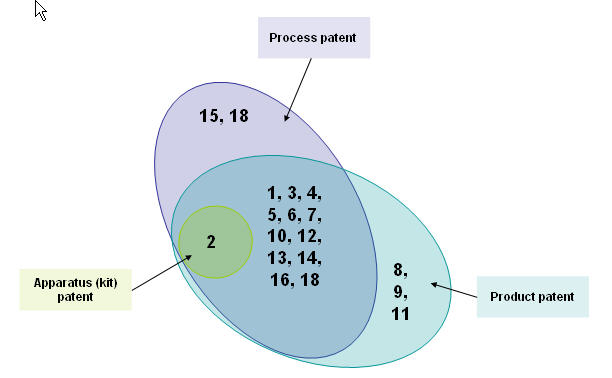
Distribution of patents
By patent types
By key ingredients
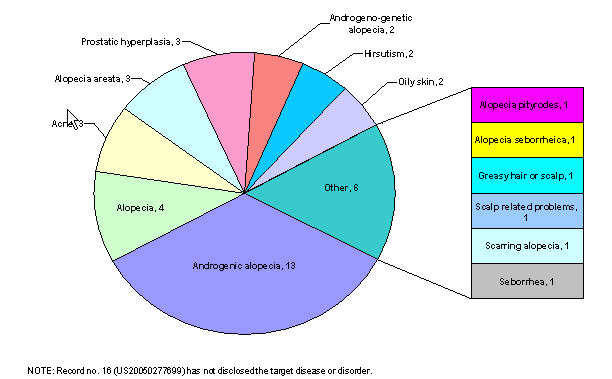
By target disease
Key ingredients vs. Target disease
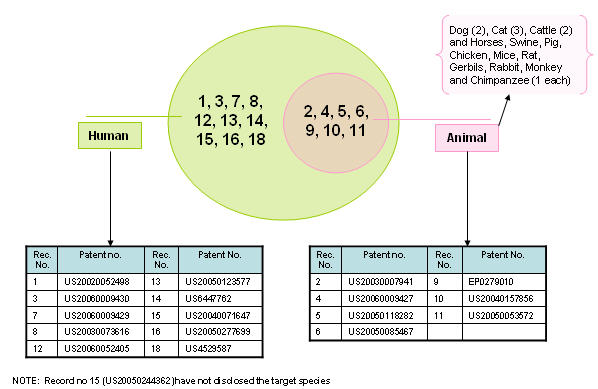
Target species
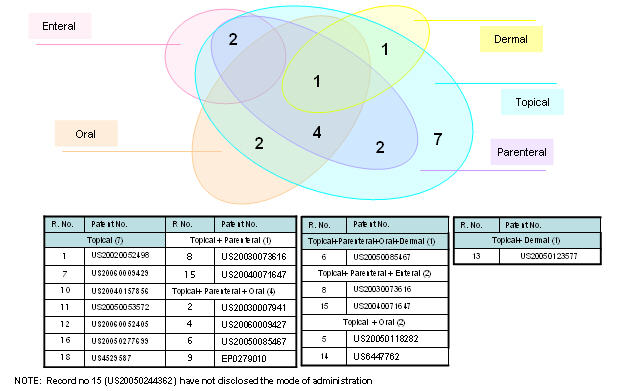
Mode of administration
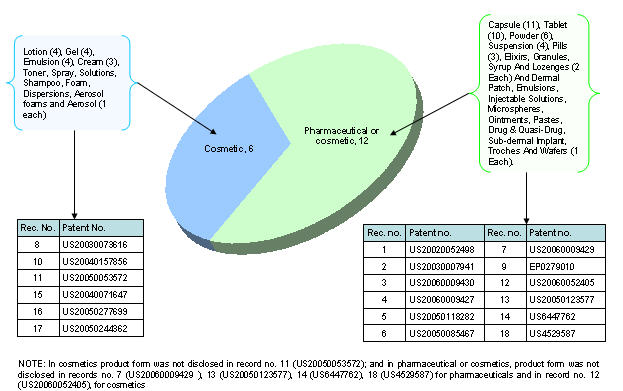
Product type vs. Product form
Patents by target diseases
| Target disease/ disorder | Patent no. | Rec. no. |
|---|---|---|
| Alopecia areata, alopecia pityrodes or alopecia seborrheica, or androgenic alopecia (i.e. male pattern baldness) | US20020052498 | 1 |
| Alopecia areata, male pattern baldness and female pattern baldness | US20030007941 | 2 |
| Androgenic alopecia (i.e. male pattern baldness), prostatic hyperplasia or both. | US20060009430 | 3 |
| Inappropriate activation of the androgen receptor, acne, oily skin, alopecia | US20060009427 | 4 |
| Prostatic hyperplasia, prostatic cancer, hirsutism, acne, male pattern baldness, seborrhea, and other diseases related to androgen hyperactivity | US20050118282 | 5 |
| Alopecia, acne, oily skin, prostrate cancer, hirsutism, and benign prostate hyperplasia | US20050085467 | 6 |
| Androgen-associated hair loss and androgen-skin related disorders. | US20060009429 | 7 |
| Androgenetic or androgenic alopecia or androgeno-genetic alopecia | US20030073616 | 8 |
| Diseases caused by testosterone (male-pattern alopecia) | EP0279010 | 9 |
| Alopecia areata, female pattern hair loss, hair loss secondary to chemotherapy or radiation treatment, stress-related hair loss, self-induced hair loss, scarring alopecia, and alopecia in non-human mammal | US20040157856 | 10 |
| Male pattern alopecia | US20050053572 | 11 |
| Alopecia, androgenic alopecia | US20060052405 | 12 |
| Hair loss | US20050123577 | 13 |
| Male pattern alopecia | US6447762 | 14 |
| Androgenetic, androgenic or androgenogenetic alopecia | US20040071647 | 15 |
| Curing other scalp related problems | US20050244362 | 16 |
Patents by application
List of patents
Signaling Pathway and linkages
Pathways associated with hair matric cell
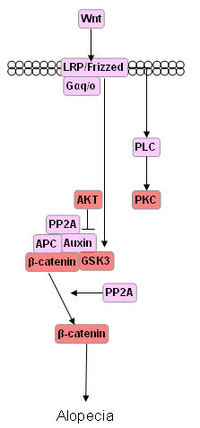
Molecular mediators of hair follicle embryogenesis: Identification of the molecular pathways controlling differentiation and proliferation in mammalian hair follicles provides the crucial link to understanding the regulation of normal hair growth, the basis of hereditary hair loss diseases, and the origin of follicle-based tumors. Homeobox (hox), hedgehog (hh), patched (ptc), wingless (wg}/wnt, disheveled (dsh), engrailed (en), Notch 1 and armadillo/B-catenin genes are all critical for hair follicle.
- Wnt pathway: Maintains hair-inducing activity of the dermal papilla.
- Hedgehog pathway: Sonic hedgehog (SHH) signaling plays a critical role in hair follicle development. Sonic hedgehog gene. Sonic hedgehog, SHH for short, helps guide hair follicles from a resting stage into growth activity. SHH is particularly important in the embryonic formation of hair follicles.
- STAT pathway
- TGF beta/BMP Pathway: Bone morphogenetic protein (BMP) signaling have been implicated in the regulation of both proliferation and differentiation in the hair follicle. BMP2 is expressed in the embryonic ectoderm, but then localizes to the early hair follicle placode and underlying mesenchyme. BMP4 is expressed in the early dermal condensate. Research results show that BMPs are a key component of the signaling network controlling hair development and are required to induce the genetic program regulating hair shaft differentiation in the anagen hair follicle. Transforming growth factor beta (TGF-beta), inhibits mitogen - induced dermal papilla cell proliferation
- FGF Pathway: Fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) potentiate the growth of dermal papilla cells. It is proposed that these proteins increase the synthesis of stromelysin (an enzyme, matrix metalloproteinase) which acts on the papilla cells and accelerates their growth.
- MAPK Pathway: Mitogen-activated protein kinase (MAPK) activation, increases keratinocyte turnover.
- NOTCH Pathway: Notch-1 is expressed in ectodermal-derived cells of the follicle, in the inner cells of the embryonic placode and the follicle bulb, and in the suprabasal cells of the mature outer root sheath. Delta-1, one of the three ligands is only expressed during embryonic follicle development and is exclusive to the mesenchymal cells of the pre-papilla located beneath the follicle placode, and appears to promote and accelerate placode formation, while suppressing placode formation in surrounding cells. Other ligands, Serrate 1 and Serrate 2, are expressed in matrix cells destined to form the inner root sheath and hair shaft.
| Patent no. | Key compound | Players of inhibition |
|---|---|---|
| US6664247 | Pyrazole compounds | GSK3 |
| US6989385 | Pyrazole compounds | GSK3 |
| WO2005012256 | Pyrazole compounds | CDK,GSK3 |
| US6974819 | Pyrimidine derivative | GSK3 |
| US6743791 | Heterocyclic compounds | AKT3, GSK-3, ERK2 |
| US20050277773 | Pyrrolo[3,2-d]pyrimidine derivatives | GSK3 |
| US20040072836 | Aza-oxindole derivatives | GSK3, AKT, PKC |
| EP1477489 | Pyrrolopyrimidine derivatives | GSK3 |
| WO0056710 | 3-(Anilinomethylene) oxindoles | GSK3, AKT, PKC |
| WO2003011287 | Pyrazolon derivatives | GSK3, β-catenin |
| US6924141 | Lithium chloride, Wnt3/4/ 7 | β-catenin, GSK3, Wnt |
| US6706685 | Peptide sequence | β-catenin |
| US6683048 | Peptide sequence | α-catenin, β-catenin |
| US6677116 | Peptide sequence LXXLL | β-catenin |
| US6303576 | Peptide sequence LXXLL | β-catenin |
Role of Pyrazole compounds in Wnt Pathway
Pyrazole
- Pyrazole (C3H4N2) refers both to the class of simple aromatic ring organic compounds of the heterocyclic series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions and to the unsubstituted parent compound. Being so composed and having pharmacological effects on humans, they are classified as alkaloids although they are not known to occur in nature.
- Pyrazoles are produced synthetically through the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation
- Pyrazoles are used for their analgesic, anti-inflammatory, antipyretic, antiarrhythmic, tranquilizing, muscle relaxing, psychoanaleptic, anticonvulsant, monoamineoxidase inhibiting, antidiabetic and antibacterial activities.
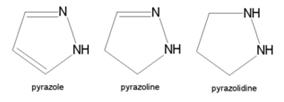
- Structurally related compounds are pyrazoline and pyrazolidine.
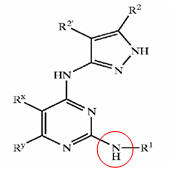
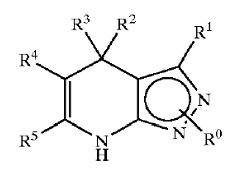
GSK3 inhibition by pyrazole compounds
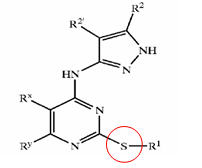
| US6989385 | US6664247 | WO2005012256 |
|---|---|---|
| R1=T-Ring D, wherein
T is a valence bond and Ring D = 5-6 membered aryl or heteroaryl ring; R2 = hydrogen or C1-4 aliphatic and R2'= hydrogen; R3 = -R, -OR, or -N(R4)2, wherein R = hydrogen, C1-6 aliphatic, 5-6 membered heterocyclyl, phenyl, or 5-6 membered heteroaryl, and L is -O-, -S-, or -NH-; and Ring D is substituted by up to three substituents selected from -halo, -CN, -NO2, -N(R4)2, optionally substituted C1-6 aliphatic group, -OR, -C(O)R, -CO2R, -CONH(R<4>), -N(R4)COR, -N(R4)CO2R, -SO2N(R4)2, -N(R4)SO2R, -N(R6)COCH2N(R4)2, -N(R6)COCH2CH2N(R4)2, or -N(R6)COCH2CH2CH2N(R4)2, wherein R = hydrogen, C1-6 aliphatic, phenyl, 5-6 membered heteroaryl ring, or 5-6 membered heterocyclic ring |
X = R1-A-NR4- or a 5- or 6-membered carbocyclic or heterocyclic ring; A is a bond, S02, C=O, NRg(C=O) or O(C=O) wherein Rg is hydrogen or C1-4 hydrocarbyl optionally substituted by hydroxy or C1-4 alkoxy; Y is a bond or an alkylene chain of 1, 2 or 3 carbon atoms in length;
R1 is hydrogen; carbocyclic or heterocyclic group having from 3 to 12 ring members; or C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from halogen (e.g. fluorine), hydroxy, C1-4 hydrocarbyloxy, amino, mono- or di-C1-4 hydrocarbylamino, and carbocyclic or heterocyclic groups having from 3 to 12 ring members, and wherein 1 or 2 of the carbon atoms of the hydrocarbyl group may optionally be replaced by an atom or group selected from 0, S, NH, SO, S02; R2 is hydrogen; halogen; C1-4 alkoxy (e.g. methoxy); or a C1-4 hydrocarbyl group optionally substituted by halogen (e.g. fluorine), hydroxyl or C1-4 alkoxy (e.g. methoxy); R3 is selected from hydrogen and carbocyclic and heterocyclic groups having from 3 to 12 ring members; and R4 is hydrogen or a C1-4 hydrocarbyl group optionally substituted by halogen (e.g. fluorine), hydroxyl or C1-4 alkoxy (e.g. methoxy). |
X is a groupR1-A-NR4-or a 5-or 6-membered carbocyclic or heterocyclic ring;
A is a bond,SO2, C=O, NRg (C=O) or O(C=O) wherein Rg is hydrogen orC14 hydrocarbyl optionally substituted by hydroxy or C1-4 alkoxy;Y is a bond or an alkylene chain of 1,2 or 3 carbon atoms in length;R'is hydrogen; a carbocyclic or heterocyclic group having from 3 to 12 ring members; or a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from halogen (e. g. fluorine), hydroxy, C1-4 hydrocarbyloxy, amino, mono-ordi-Cl 4 hydrocarbylamino, and carbocyclic or heterocyclic groups having from 3 to 12 ring members, and wherein 1 or 2 of the carbon atoms of the hydrocarbyl group may optionally be replaced by an atom or group selected fromO, S, NH, SO, SO2 ;R2 is hydrogen; halogen;C14 alkoxy (e. g. methoxy); or aC14 hydrocarbyl group optionally substituted by halogen (e. g. fluorine), hydroxyl orC14 alkoxy (e. g. methoxy);R3 is selected from hydrogen and carbocyclic and heterocyclic groups having from 3 to 12 ring members; andR4 is hydrogen or a C1-4 hydrocarbyl group optionally substituted by halogen (e. g. fluorine), hydroxyl or C1-4 alkoxy (e. g. methoxy). |
Inhibition by amine derivatives
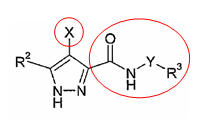
Patent Number: US6989385 Applicant: Vertex Pharmaceuticals Incorporated Title: Pyrazole compounds useful as protein kinase inhibitors
Basic Structure:
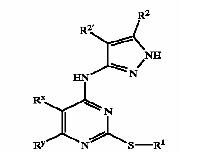
Derivatives of pyrimidine-pyrazole amine disclosed in US6989385 patent
Patent Number: US7008948 Applicant: Vertex Pharmaceuticals Incorporated Title: Fused pyrimidyl pyrazole compounds useful as protein kinase inhibitors
Basic Structure
Derivatives of pyrimidine-pyrazole amine disclosed in US7008948 patent
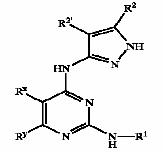
Patent Number: US6977262 Assignee: Mitsubishi Pharma Corporation Title: Dihydropyrazolopyridine compounds and pharmaceutical use thereof
Basic Structure:
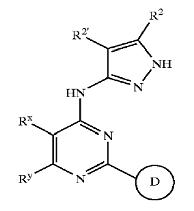
Derivatives of pyrimidine-pyrazole amine disclosed in US6977262 patent
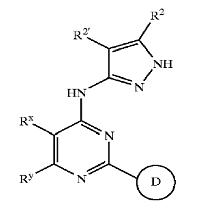
Patent Number: US6664247 Assignee: Vertex Pharmaceuticals Incorporated Title: Pyrazole compounds useful as protein kinase inhibitors
Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in US6664247 patent
Patent Number: US2004224944 Assignee: VERTEX PHARMACEUTICALS INC Title: Pyrazole compounds useful as protein kinase inhibitors
Basic Structure:
Derivatives of pyrimidine-pyrazole amine disclosed in US2004224944 patent
GSK-3 Inhibition Mechanism - Phosphorylation
- GSK-3 inhibition targets treatment of chemotherapy-induced alopecia source
- In the canonical Wnt signaling cascade, adenomatous polyposis coli (APC), axin, and GSK3 constitute the so-called destruction complex, which controls the stability of beta-catenin. It is generally believed that four conserved Ser/Thr residues in the N terminus of beta-catenin are the pivotal targets for the constitutively active serine kinase GSK3. GSK3 covalently modifies beta-catenin by attaching phosphate groups (from ATP) to serine, and threonine residues. In so doing, the functional properties of the protein kinase’s substrate (beta-catenin) are modified.
- In the absence of Wnt signals, glycogen synthase kinase (GSK) is presumed to phosphorylate the N-terminal end of beta-catenin, thus promote degradation of beta-catenin and subsequent ubiquitination and proteasomal targeting.
- Exposure of cells to Wnts leads to inactivation of GSK-3 through an as yet unclear mechanism.The phosphoprotein Dishevelled is required, after receptor-ligand interaction, to transduce the signal that results in the inactivation of GSK-3. As a result, beta-catenin is dephosphorylated and escapes the ubiqduitylation-dependent destruction machinery.
- Unphosphorylated beta-catanin accumulates in the cytoplasm and translocates to the nucleus, where it can associate with the TCF/LEFs and become a transcriptional transactivator.
Key points
- Beta-catenin phosphorylation at serine 45 (Ser45), threonine 41 (Thr41), Ser37, and Ser33 is critical for beta-catenin degradation. source
- Regulation of beta-catenin phosphorylation is a central part of the canonical Wnt signaling pathway. source
- Ser-X-X-X-Ser (X is any amino acid) motif is obligatory for beta-catenin phosphorylation by GSK3.source
- Beta-catenin phosphorylation/degradation and its regulation by Wnt can occur normally in the absence of Thr41 as long as the Ser-X-X-X-Ser motif/spacing is preserved. [httSp://pubs.acs.org/cgi-bin/abstract.cgi/bichaw/2006/45/i16/abs/bi0601149.html source]
GK3 Inhibition:
- GSK3 is regulated by phosphorylation.
- Phosphorylation of GSK3beta on Ser9 (Ser21 in GSK3alpha) by protein kinase B (PKB) causes its inactivation is the primary mechanism responsible for growth factor inhibition of this kinase. Activation of GSK3beta is dependent upon the phosphorylation of Tyr216 (Tyr279 in GSK3alpha). Upon activation, it has been shown to phosphorylate a number of different cellular proteins, including p53, c-Myc, c-Jun, heat shock factor-1 (HSF-1), beta-catenin and cyclin D1. source
- GSK3 is inhibited by phosphorylation of serine-9 or serine-21 in GSK3beta and GSK3alpha, respectively. source
- GSK3’s substrate specificity is unique in that phosphorylation of substrate only occurs if a phosphoserine or phosphotyrosine is present four residues C-terminal to the site of GSK phosphorylation. source
- A phosphorylation cascade starts from GSK3 itself and initiates it in beta-catenin. source
- Thus our goal is to stop the phosphorylation of the serine and threonine residue of GSK3.
- The figure below illustrates the phosphorylation mechanism of serine and threonine by ATP.
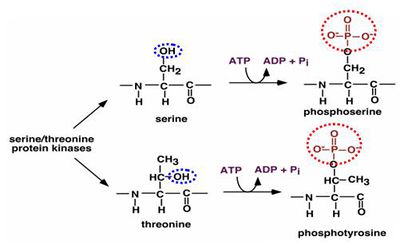
- We can't stop conversion of ADP to ATP that relaseas Phosphorous group causing Phosphorylation.
- We can only block the oxygen atom on serine and threonine as a result which will in turn stop Phosphorylation.
- The two probable ways of blocking the oxygen atom are (a) As oxygen is a Lewis acid with strong electron donating capacity, so usually a strong electron pair acceptor can easily bind to oxygen atom and preventing phosphorylation or (b) breaking of the -OH bond with the carbon atom.
Serine - pyrazole reaction
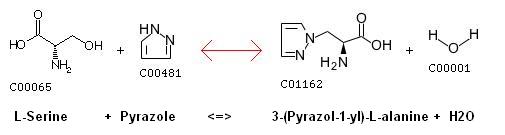
- The T-loop of GSK-3 is tyrosine phosphorylated at Y216 and Y279 in GSK-3b and GSK-3a, respectively, but not threonine phosphorylated. Y216/Y279 phosphorylation could play a role in forcing open the substrate (e.g, beta-catanin)-binding site.
- Thus, T-loop tyrosine might facilitate substrate phosphorylation but is not strictly required for kinase activity.
- Stimulation of cells with pyrazole compounds cause inactivation of GSK-3 through phosphorylation (S9 of GSK-3 beta and S21 of GSK-3 alpha), which inhibits GSK-3 activity. Thus leading to dephosphorylation of substrates (e.g., beta-catanin) resulting in their functional activation and consequent increased hair follicle morphogenisis.
- Phosphorylation of S9/S21 creates a primed pesudosubstrate that binds intramolecularly to the positively charged pocket of the GSK-3. This folding precludes phosphorylation of substrates (eg., beta-catanin) because the catalytic groove is occupied. The mechanism of inhibition is competitive.
- A consequence of this is that primed substrates, in high enough concentrations, out-compete the pesudosubstrate and thus become phosphorylated.
- Thus, small molecule inhibitors modeled to fit in the positively charged pocket of the GSK-3 kinease domain could potentially be very effective for selective inhibition of primed substrates.
Proposed mechanisms to regulate GSK-3 source
- inactivation of GSK-3 through serine phosphorylation
- activation of GSK-3 through tyrosine phosphorylation
- inactivation of GSK-3 through tyrosine dephosphorylation
- Covalant modifications of substrates through priming phosphorylation
- inhibition or facilation of GSK-3 mediated substrate phosphorylation thriugh interation of GSK-3 with binding or scaffolding proteins
- targeting of GSK-3 to different subcellular localizations
- differential usage of isoforms or splice variants to alter subcellular localization or substrate specificity
- integration of parellel signals conveyed by a signal stimulus.
Key Finding
- Pyrazole compounds with inhibition constant (Ki) of <0.1 mM are a good starting point for developing molecules that can inhibit serine/threonine protein kinase (such as GSK-3) and the proteins they help to regulate. source
Pathway associated with anti-androgen
Structure-Activity Relationships(SARs)
Error code: 134
Pathway associated with Minoxidil (vasodialators)
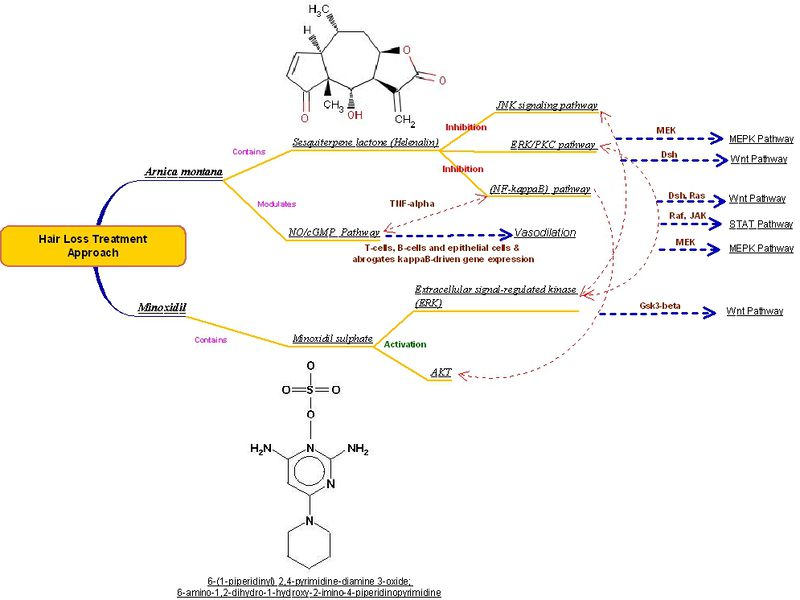
Minoxidil is a well know drug used for the treatment of alopecia. A co-relation between Sesquiterpene lactone (Helenalin) produced from Arnica montana and Minoxidil is illustrated in the figure below. Arnica montana being a Vasodilator act on NO/cGMP Pathway through T-cells, B-cells and epithelial cells & abrogates kappa B-driven gene expression
Conclusions
- Hair loss medication is a very active area of research and intellectual property development.
- One of the most promising areas of development is the area of Anti-androgens.
- The top companies are Merck, L’Oreal and Smithkline.
Questions Dolcera Answers
What’s hot?
- What compositions/ approaches are the most promising?
- What can I license?
- Can you map blockbuster products to their patents?
Can you save me some time?
- What combinations/ compounds have already been tried?
- Is any empirical data available?
- Can you tell me the side effects?
Where should I focus my R&D investment?
- What are the most promising approaches?
- Where’s the ‘white space’ for me to play in?
Any hints for research?
- Are there any combinations I could develop?
What should I do in this geography?
- What are my competitors up to in this geography?
- What are my strengths/ weaknesses here?
What’s my competition up to?
- What’s my top competitor investing in?
- Are there any loopholes in their patents?
- When are their patents expiring?
- Will a competitor emerge from nowhere and surprise me?
- What are the crowded areas?
How do I play defense?
- What should my blocking/reactive strategies be?