Difference between revisions of "Ureteral Stent"
| Line 1: | Line 1: | ||
| + | '''This is a landscape report on the Ureteral stent market, including key company profiles, products, patents and relevant clinical trails. | ||
| + | ''' | ||
| + | |||
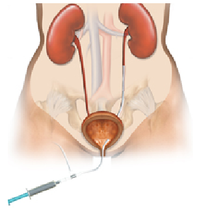
| + | *'''''What is it?''''' A ureteral stent is a specially designed hollow tube, made of a flexible plastic material that is placed in the ureter. | ||
| + | |||
| + | *'''''Need for a ureteral stent:''''' In patients who have, or might have, an obstruction (blockage) of the kidney, an internal drainage tube called a ‘stent’ is commonly placed in the ureter, the tube between the kidney and the bladder. This is placed there in order to prevent or temporarily relieve the obstruction. | ||
| + | |||
| + | ==Background== | ||
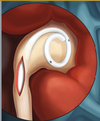
| + | [[Image:Ureteral_Stent.png|thumb|200px|<center>[http://www.pnnmedical.com/urology/professionals/products/memokath%E2%84%A2-051-ureter.aspx '''Ureteric Stent''']</center>]] | ||
| + | |||
| + | Ureteral stents are used in urological surgery to maintain patency of the ureter to allow urine drainage from the renal pelvis to the bladder. These devices can be placed by a number of different endourological techniques. They are typically inserted through a cystoscope and may also be inserted intraoperatively. Indwelling ureteral stents help to reduce complications and morbidity subsequent to urological and surgical procedures. Frequently, ureteral stents are used | ||
| + | to facilitate drainage in conjunction with Extracorporeal Shock Wave Lithotripsy (ESWL) and after endoscopic procedures. They are also used to internally support anastomoses and prevent urine leakage after surgery. Ureteral stenting may almost eliminate the urological complications of renal transplantation. | ||
| + | An antimicrobial ureteral stent, which inhibits encrustation and bacterial colonization while maintaining patient comfort. | ||
| + | * Ureteral stent: resists migration, resists fragmentation, is kink resistant and radiopaque. | ||
| + | * Bacterial colonization: antimicrobial activity for up to two weeks. | ||
| + | * Patient Comfort: stent has a low coefficient of friction (value) for ease of insertion and will soften on implant at body temperature to maintain patient comfort. | ||
| + | |||
| + | '''[[more on background...]]''' | ||
| + | |||
| + | =Market Overview= | ||
| + | |||
| + | Market for ureteral stent can be analyzed by estimating market for each of Ureteral Stent’s fundamental use. Other uses of Ureteral Stent include Post-surgical swelling/infection of uterus, Active kidney infection etc. | ||
| + | |||
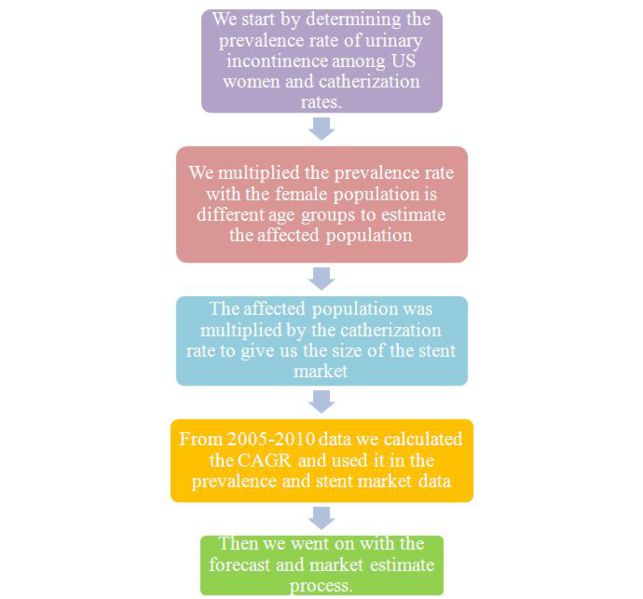
| + | =='''Methodology'''== | ||
| + | ''' [[Image:Method1.JPG|700px]]''' | ||
| + | |||
| + | =='''Kidney Stones Market'''== | ||
| + | |||
| + | There are four main types of treatments available for curing kidney stones based on type of Kidney Stone, listed as follows | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Sr. No.'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Treatment'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Indications'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Requirement of Ureteral Stent'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''% of cases in general'''</font> | ||
| + | |- | ||
| + | |align = "right" bgcolor = "#DBE5F1"|1 | ||
| + | |bgcolor = "#DBE5F1"|Lithotripsy | ||
| + | |bgcolor = "#DBE5F1"|Radiolucent calculi, Renal stones <nowiki><</nowiki>2 cm, Ureteral stones <nowiki><</nowiki>1 cm | ||
| + | |bgcolor = "#DBE5F1"|No | ||
| + | |align = "right" bgcolor = "#DBE5F1"|49.07%<nowiki>*</nowiki> | ||
| + | |- | ||
| + | |align = "right"|2 | ||
| + | |Ureteroscopy | ||
| + | |Ureteral stones | ||
| + | |Yes | ||
| + | |align = "right"|15.48%<nowiki>*</nowiki> | ||
| + | |- | ||
| + | |align = "right" bgcolor = "#DBE5F1"|3 | ||
| + | |bgcolor = "#DBE5F1"|Ureterorenoscopy | ||
| + | |bgcolor = "#DBE5F1"|Renal stones <nowiki><</nowiki>2 cm | ||
| + | |bgcolor = "#DBE5F1"|Yes | ||
| + | |align = "right" bgcolor = "#DBE5F1"|0.47%<nowiki>*</nowiki> | ||
| + | |- | ||
| + | |align = "right"|4 | ||
| + | |Percutaneous nephrolithotomy | ||
| + | |Renal stones <nowiki>></nowiki>2 cm, Proximal ureteral stones <nowiki>></nowiki>1 cm | ||
| + | |No | ||
| + | |align = "right"|34.98%<nowiki>*</nowiki> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | <nowiki>*</nowiki>Dolcera Estimate 2011 | ||
| + | |||
| + | As seen in table ureteral stent is required for Ureteroscopy and Ureterorenoscopy which together constitute for around 16% of Kidney stone cases. | ||
| + | |||
| + | Total Number of Kidney Stone Cases in US in 2006: 166,000 | ||
| + | |||
| + | Approximate Stents required in 2006 = 166,000<nowiki>*</nowiki>0.16=26560 | ||
| + | |||
| + | =='''Kidney Transplant Market'''== | ||
| + | |||
| + | Every kidney transplant operation required ureteral stent to be placed in Patient<nowiki>’</nowiki>s body for few days till newly placed kidney adapts to Patients<nowiki>’</nowiki> body | ||
| + | |||
| + | Total No. of Kidney Transplants done in United States in 2007 are approx. 17,513 | ||
| + | |||
| + | Total Market for Ureteral Stents in 2007 is 17,513 | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="54%" | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Year'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''No. of Kidney Transplants'''</font> | ||
| + | |- | ||
| + | |align = "right"|'''2007''' | ||
| + | | 17,513 | ||
| + | |- | ||
| + | |align = "right"|'''2006''' | ||
| + | | 18,056 | ||
| + | |- | ||
| + | |align = "right"|'''2005''' | ||
| + | | 17,443 | ||
| + | |- | ||
| + | |align = "right"|'''2000''' | ||
| + | | 14,611 | ||
| + | |- | ||
| + | |align = "right"|'''1995''' | ||
| + | | 12,160 | ||
| + | |- | ||
| + | |align = "right"|'''1990''' | ||
| + | | 10,029 | ||
| + | |- | ||
| + | |align = "right"|'''1985''' | ||
| + | | 7,504 | ||
| + | |- | ||
| + | |align = "right"|'''1980''' | ||
| + | | 3,785 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | =='''Urinary Incontinence Market'''== | ||
| + | |||
| + | '''Inpatient hospital stays:'''The estimated number of hospital admissions among adults ages 18 or older with urinary incontinence listed as a diagnosis: <br>'''(2000):''' 47,802 hospital stays (1,332 men; 46,470 women) | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="40%" | ||
| + | |align = "center" bgcolor = "#5CB3FF" colspan = "2"|'''Prevalence of Urinary Incontinece in US (Women)''' | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|'''Age (in years)''' | ||
| + | |'''Population with Urinary Incontinence (in %)''' | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|30-39 | ||
| + | |align = "center"|28 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|40-49 | ||
| + | |align = "center"|41 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|50-59 | ||
| + | |align = "center"|48 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|60-69 | ||
| + | |align = "center"|51 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|70-79 | ||
| + | |align = "center"|55 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|≥ 80 | ||
| + | |align = "center"|54 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="102%" | ||
| + | |align = "center" colspan = "6"|'''Estimated cases for ureteral stents in US, 2010''' | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|Age groups | ||
| + | |bgcolor = "#5CB3FF"|A) Female population (Million) | ||
| + | |bgcolor = "#5CB3FF"|B) Prevalence rate in female (%) | ||
| + | |bgcolor = "#5CB3FF"|C=A<nowiki>*</nowiki>B Affected population<br>(Million) | ||
| + | |bgcolor = "#5CB3FF"|D) Catherization rate (%) | ||
| + | |bgcolor = "#5CB3FF"|E=C<nowiki>*</nowiki>D<br>Stent market based on catherization | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|30-39 | ||
| + | |align = "right"|20.10 | ||
| + | |align = "right"|28 | ||
| + | |align = "right"|5.62 | ||
| + | |align = "right"|0.043 | ||
| + | |2420.42 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|40-49 | ||
| + | |align = "right"|21.99 | ||
| + | |align = "right"|41 | ||
| + | |align = "right"|9.01 | ||
| + | |align = "right"|0.123 | ||
| + | |11092.83 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|50-59 | ||
| + | |align = "right"|21.50 | ||
| + | |align = "right"|48 | ||
| + | |align = "right"|10.32 | ||
| + | |align = "right"|0.124 | ||
| + | |12800.37 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|60-69 | ||
| + | |align = "right"|15.32 | ||
| + | |align = "right"|51 | ||
| + | |align = "right"|7.81 | ||
| + | |align = "right"|0.160 | ||
| + | |12503.68 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|70-79 | ||
| + | |align = "right"|9.16 | ||
| + | |align = "right"|55 | ||
| + | |align = "right"|5.04 | ||
| + | |align = "right"|0.172 | ||
| + | |8674.44 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|≥ 80 | ||
| + | |align = "right"|7.15 | ||
| + | |align = "right"|54 | ||
| + | |align = "right"|3.86 | ||
| + | |align = "right"|0.044 | ||
| + | |1699.48 | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|Total | ||
| + | |align = "right"|95.25 | ||
| + | | | ||
| + | |align = "right"|41.69 | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |} | ||
| + | * '''Catherization rate''' depicts the actual number of people going in for ureteral stents. | ||
| + | |||
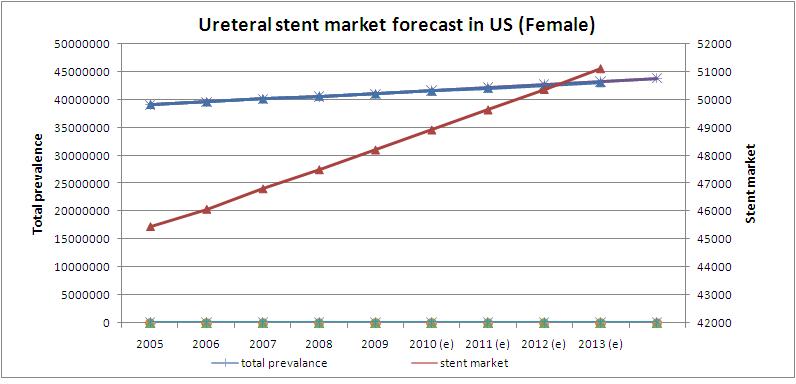
| + | * Prevalence rate in US women is growing at a CAGR of 1.26% | ||
| + | |||
| + | Assuming that each admission in hospital required one ureteral stent, market from Urinary Incontinence is around 47,802 stents per year | ||
| + | |||
| + | '''Total Market for Ureteral Stent''' | ||
| + | Following table displays approximate stent market per year | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="64%" | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Category'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''No. of Stents Required'''</font> | ||
| + | |- | ||
| + | |bgcolor = "#DBE5F1"|Stent Market for Kidney Stones | ||
| + | |align = "center" bgcolor = "#DBE5F1"|26560 | ||
| + | |- | ||
| + | |Stent Market for Kidney Transplant | ||
| + | |align = "center"|17513 | ||
| + | |- | ||
| + | |bgcolor = "#DBE5F1"|Stent Market for Urinary Incontinence | ||
| + | |align = "center" bgcolor = "#DBE5F1"|47802 | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">Total</font> | ||
| + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">91875</font> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | * Ureteral stent market is growing at a CAGR of 1.58% | ||
| + | |||
| + | |||
| + | |||
| + | =='''Ureteral stent market forecast in US (women)'''== | ||
| + | ''' | ||
| + | [[Image:stent_forcast.jpeg]] | ||
| + | ''' | ||
| + | <br/> | ||
| + | <br/> | ||
| + | [[media:4887766.xlsx|Detailed_calculation_workbook]] | ||
| + | |||
| + | |||
| + | '''[[more on market overview...]]''' | ||
| + | |||
| + | =Interactive Mind Map= | ||
| + | [[Image:legend10.png|right|thumb|300px|'''Legend''']] | ||
| + | *''Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy'' | ||
| + | |||
| + | *''Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard'' | ||
| + | |||
| + | *''Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap'' | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |||
| + | |<mm>[[Mmap905(1 (new1).1).mm|flash|Ureteral Stent mindmap|600pt]]</mm> | ||
| + | |||
| + | |} | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |||
| + | |<mm>[[ureteral_stent_mapping.mm.mm|flash|Ureteral Stent mindmap|600pt]]</mm> | ||
| + | |||
| + | |} | ||
| + | |||
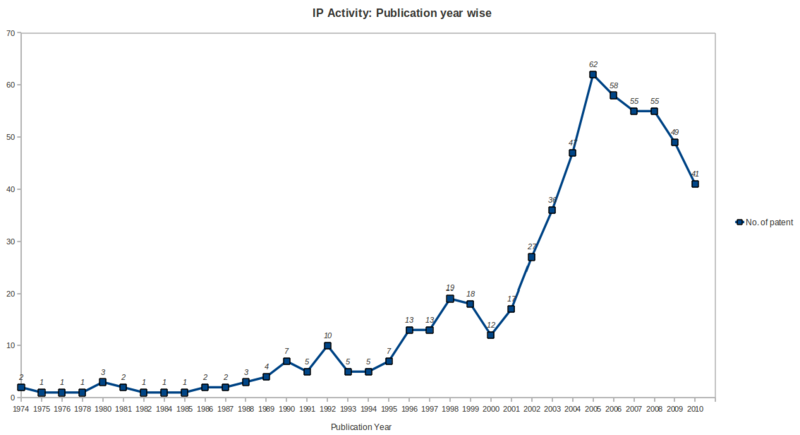
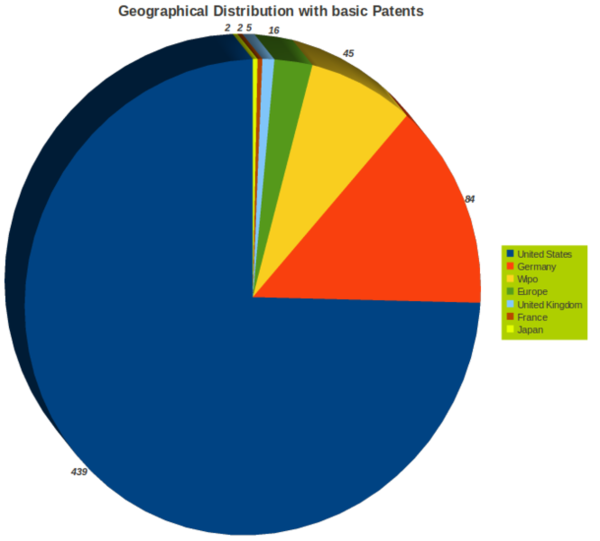
| + | =Patents= | ||
| + | ==Patent Search Strategy== | ||
| + | [[Image:Patent Search Strategy.jpg|700px]] | ||
| + | |||
| + | ==Dolcera Patent Analysis== | ||
| + | |||
| + | {|border "1" style="border-spacing:0;" | ||
| + | | rowspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''S.No. '''</font></center> | ||
| + | | rowspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Patent/Publication No.'''</font></center> | ||
| + | | rowspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Publication Date''' (mm/dd/yyyy)</font></center> | ||
| + | | rowspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Assignee/Applicant'''</font></center> | ||
| + | | rowspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Title'''</font></center> | ||
| + | | colspan="2" style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Dolcera Analysis'''</font></center> | ||
| + | |- | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center>'''Problem'''</center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center>'''Solution'''</center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 1 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220090187254%22.PGNR.&OS=DN/20090187254&RS=DN/20090187254 US20090187254A1] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 07/23/2009 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| BOSTON SCIENTIFIC SCIMED, INC. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| UROLOGICAL MEDICAL DEVICES FOR RELEASE OF UROLOGICALLY BENEFICIAL AGENTS | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| This invention provides a ueteral stent which release one or more urologically beneficial agents in effective amounts to reduce the problem of encrustation and pain related to stent. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| 2 | ||
| + | | style="padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220090171465%22.PGNR.&OS=DN/20090171465&RS=DN/20090171465 US20090171465A1] | ||
| + | | style="padding:0.079cm;"| 07/02/2009 | ||
| + | | style="padding:0.079cm;"| Boston Scientific Scimed, Inc. | ||
| + | | style="padding:0.079cm;"| Polymeric Regions For Implantable Or Insertable Medical Devices | ||
| + | | style="padding:0.079cm;"| In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | ||
| + | | style="padding:0.079cm;"| This invention provides a ureteral stent with a coating of a material(EVA) that reduces the discomfort caused by the stent during implantation. | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 3 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220080097349%22.PGNR.&OS=DN/20080097349&RS=DN/20080097349 US20080097349A1] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 04/24/2008 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Boston Scientific Scimed, Inc. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Partially soluble implantable or insertable medical devices | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Here the device (Stent) is provided with at least one surface that contains one or more depressions, which are at least partially filled with a soluble material. This makes stent more flexible and soft after implantation hence minimizing pain and discomfort after implantation or insertion. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| 4 | ||
| + | | style="padding:0.079cm;"| [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5554147.PN.&OS=PN/5554147&RS=PN/5554147 US5554147A] | ||
| + | | style="padding:0.079cm;"| 09/10/1996 | ||
| + | | style="padding:0.079cm;"| CApHCO, Inc. | ||
| + | | style="padding:0.079cm;"| Compositions and devices for controlled release of active ingredients | ||
| + | | style="padding:0.079cm;"| During implantation of ureteral stent,pain and discomfort are the problem with previous stents. Also microbial or bacterial infection at the site of implantation was the problem. | ||
| + | | style="padding:0.079cm;"| In this invention stent is povided with a pH sensitive coating that includes antimicrobial agent to stop the infection. This coating also has the hydrogel property which Upon swelling, the hydrogel's coefficient of friction is reduced, and the polymer becomes slippery, this mechanism reduces the discomfort. | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 5 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6468649.PN.&OS=PN/6468649&RS=PN/6468649 US6468649B1] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 10/22/2002 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Scimed Life Systems, Inc. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Antimicrobial adhesion surface | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| In prior art problem was the lack of the device which can remain in vivo for extended periods of time without losing its antimicrobial efficacy and which can provides protection against bacterial and fungal organisms for extended periods of time without leaching substances into a patient. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| This invention provides a medical device with a hydrophobic coating that inhibits the growth of microbes for extended period of time. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| 6 | ||
| + | | style="padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220030153983%22.PGNR.&OS=DN/20030153983&RS=DN/20030153983 US20030153983A1] | ||
| + | | style="padding:0.079cm;"| 08/14/2003 | ||
| + | | style="padding:0.079cm;"| Scimed Life Systems, Inc. | ||
| + | | style="padding:0.079cm;"| Implantable or insertable medical device resistant to microbial growth and biofilm formation | ||
| + | | style="padding:0.079cm;"| In previous attempts to develope a ureteral stent with coating the problem was the formation of biofilm and microbial growth. | ||
| + | | style="padding:0.079cm;"| The device of the present invention, therefore, overcomes the disadvantages associated with the use of coatings as discussed above, and provides a reduced risk of biofilm fouling that eventually results in encrustation, occlusion and failure of the device. In this invention medical device comprises at least one biocompatible matrix polymer region and bioactive agents comprising an antimicrobial agent and a microbial attachment/biofilm synthesis inhibitor. | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 7 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220030139800%22.PGNR.&OS=DN/20030139800&RS=DN/20030139800 US20030139800A1] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 07/24/2003 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| None | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Stent assembly with therapeutic agent exterior banding | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| In earlier attempts for stent with a therapeutic property, the problem was the coating or the sheath (responsible for therapy) may covers side branch arteries, vessels, or other lumens extending from the main lumen in which the stent is installed. The sheath can reduce blood flow to or from the side branch and deliver medication into the side branch where it is unnecessary. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| This invention provides a stent assembly with exterior banding for delivery of therapeutic agents that would overcome the previous disadvantages. Also this stent assembly which avoids side branch vessel blockage by a sheath, with increased drug storage capacity and allowing delivery of different drugs at different axial and radial locations. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| 8 | ||
| + | | style="padding:0.079cm;"| [http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220040117007%22.PGNR.&OS=DN/20040117007&RS=DN/20040117007 US20040117007A1] | ||
| + | | style="padding:0.079cm;"| 06/17/2004 | ||
| + | | style="padding:0.079cm;"| STS Biopolymers, Inc. | ||
| + | | style="padding:0.079cm;"| Medicated stent having multi-layer polymer coating | ||
| + | | style="padding:0.079cm;"| The problem associated with previous stent and their implantation was thrombus formation in vicinity of stent. | ||
| + | | style="padding:0.079cm;"| The stent assembly developed in this invention consistently provide therapeutic activity from the surfaces of stents in order to reduce the incidence of restenosis and thrombus formation after coronary stenting procedures in the clinic. | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 9 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://www.wipo.int/pctdb/en/wo.jsp?WO=1990013332 WO1990013332A1] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| 11/15/1990 | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| CEDARS-SINAI MEDICAL CENTER | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| STENT WITH SUSTAINED DRUG DELIVERY | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Problem was to provide an intravascular stent that preserves vessel patency, inhibits luminal narrowing and at the same time stent can deliver a pharmaceutical agent to a specific body site or organ. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| The stent assembly provided by this invention will release anticoagulants, antiplatelet drugs or drugs that inhibit excessive endothelial cell growth at the placement site, thereby preserving the vessels patency and inhibiting luminal narrowing. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| 10 | ||
| + | | style="padding:0.079cm;"| [http://www.wipo.int/pctdb/en/wo.jsp?WO=2001036008 WO2001036008A2] | ||
| + | | style="padding:0.079cm;"| 05/25/2001 | ||
| + | | style="padding:0.079cm;"| STS BIOPOLYMERS, INC. | ||
| + | | style="padding:0.079cm;"| MEDICAL DEVICES COATED WITH ELASTIC POLYMERIC MATERIAL | ||
| + | | style="padding:0.079cm;"| The lack of flexibility, expandability and lubricity of coating surface was the problem with previous insertable medical device. | ||
| + | | style="padding:0.079cm;"| This invention provides a medical device with a polymeric coating adherent to the covering, such that the coating and covering possess desirable surface characteristics such as lubricity, or lack thereof, as well as flexibility, expandability and elasticity. | ||
| + | |} | ||
| + | |||
| + | == Dolcera Dashboard == | ||
| + | [[Image:dashboard_features.png|center|750px|]] | ||
| + | |||
| + | '''Dashboard Link'''<br> | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |'''[http://client.dolcera.com/dashboard/dashboard.html?workfile_id=1008 Ureteral Stent - Dashboard] ''' | ||
| + | |width="100"|[[Image:dashboard_thumb.png|center|100px|]] | ||
| + | |- | ||
| + | |} | ||
| + | *Flash Player is essential to view the Dolcera Dashboard | ||
| + | * To access the Dashboard you have to signup. You can do so by clicking [https://www.dolcera.com/auth/index.php/login '''here'''] | ||
| + | |||
| + | ==Patent Heat Map== | ||
| + | |||
| + | |||
| + | '''[[more on patent analysis...]]''' | ||
| + | |||
| + | = Clinical Trials = | ||
| + | ==New trials == | ||
| + | |||
| + | {|border "1" style="border-spacing:0;" | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''S.No. '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Title '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Conditions '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Intervention: Device'''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Sponsors and Collaborators '''</font></center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>1</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://clinicaltrials.gov/ct2/show/NCT00250406?term=ureteral+stent&rank=1 Assessment of Drug-Eluting Ureteral Stent on Bacterial Adherence and Biofilm Formation] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|Renal Calculi, Ureteral Obstruction | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Ureteral Stent | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Lawson Health Research Institute, Boston Scientific Corporation | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>2</center> | ||
| + | | style="padding:0.079cm;"| [http://clinicaltrials.gov/ct2/show/NCT00270504?term=urethral+stent&rank=1 Memokath® 044TW Stent for Treatment of Urethral Stricture] | ||
| + | | style="padding:0.079cm;"| Urethral Stricture | ||
| + | | style="padding:0.079cm;"| Memokath stenting | ||
| + | | style="padding:0.079cm;"| Engineers & Doctors Wallsten Medical Group | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>3</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|[http://clinicaltrials.gov/ct2/show/NCT00581178?term=urologic+stent&rank=3 Study to Determine if There Are Specific Clinical Factors to Determine Stent Encrustation] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Kidney Stones | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| N\A | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| University of California, Irvine | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>4</center> | ||
| + | | style="padding:0.079cm;"| [http://clinicaltrials.gov/ct2/show/NCT00288457?term=urologic+stent&rank=14 Ureteral Stent Length and Patient Symptoms] | ||
| + | | style="padding:0.079cm;"| Kidney Stones | ||
| + | | style="padding:0.079cm;"| Ureteral Stent | ||
| + | | style="padding:0.079cm;"| Emory University | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>5</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [http://clinicaltrials.gov/ct2/show/NCT00166361?term=urologic+stent&rank=1 Drainage of Malignant Extrinsic Ureteral Obstruction Using the Memokath Ureteral Stent] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Ureteral Obstruction | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|Memokath 051 Ureteral Stent | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Mayo Clinic Engineers & Doctors Wallsten Medical Group | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>6</center> | ||
| + | | style="padding:0.079cm;"| [http://clinicaltrials.gov/ct2/show/NCT00739284?term=urologic+stent&rank=15 A Prospective Comparison Between Ureteral Stent and Nephrostomy Tube for an Urgent Drainage of Obstructed Kidney (JJVsPCN08)] | ||
| + | | style="padding:0.079cm;"| Kidney Disease | ||
| + | | style="padding:0.079cm;"| Nephrostomy tube and ureteral stent | ||
| + | | style="padding:0.079cm;"| Rabin Medical Center | ||
| + | |} | ||
| + | |||
| + | == Concluded trials == | ||
| + | {| {{table}} | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''S.No. '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Title'''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Abstract'''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Enrollment'''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Disorder'''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Conclusion'''</font></center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<center>1</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Long-term outcome of permanent urethral stents in the treatment of detrusor-sphincter dyssynergia || style="background-color:#dce6f1;padding:0.079cm;"|To evaluate the long-term efficacy of a permanently implanted urethral stent in the treatment of spinally injured patients with detrusor-sphincter dyssynergia.|| style="background-color:#dce6f1;padding:0.079cm;"|13|| style="background-color:#dce6f1;padding:0.079cm;"|Detrusor-sphincter dyssynergia|| style="background-color:#dce6f1;padding:0.079cm;"|Stenting is an effective alternative to sphincterotomy in the long-term, although secondary bladder neck obstruction is a frequent problem. | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>2</center> | ||
| + | | Nephrostomy Tube or 'JJ' Ureteric Stent in Ureteric Obstruction: Assessment of Patient Perspectives Using Quality-of-Life Survey and Utility Analysis||Upper urinary tract obstruction is often relieved by either a percutaneous nephrostomy tube (PCN) or a ureteric stent. Both can cause considerable morbidity and reduce patient's health-related quality of life (QoL). We have compared the QoL in these 2 groups.||34||Upper urinary tract obstruction||Patients with 'JJ' stents have significantly more irritative urinary symptoms and a high chance of local discomfort than patients with nephrostomy tubes (PCN). However, based on the EuroQol analysis, there is no significant difference in the gross impact on the health-related QoL or the utility between these groups indicating no patient preference for either modality of treatment. | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>3</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial.|| style="background-color:#dce6f1;padding:0.079cm;"|A randomized, prospective trial to compare the incidence of early urological complications and health care expenditures in renal transplant recipients with or without ureteral stenting.|| style="background-color:#dce6f1;padding:0.079cm;"|201|| style="background-color:#dce6f1;padding:0.079cm;"|Renal transplant recipient|| style="background-color:#dce6f1;padding:0.079cm;"|Using a ureteral stent at renal transplantation significantly decreases the early urinary complications of urine leakage and obstruction. However, there is a significant increase in urinary tract infections, primarily beyond 30 days after transplantation. Stent removal within 4 weeks of insertion appears advisable. | ||
| + | |} | ||
| + | |||
| + | ==Adverse Events== | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |align = "center" bgcolor = "#4f81bd"|<center><font color="#FFFFFF">'''S.No. '''</font></center> | ||
| + | |align = "center" bgcolor = "#4f81bd"|<center><font color="#FFFFFF">'''Brand Name'''</font></center> | ||
| + | |align = "center" bgcolor = "#4f81bd"|<center><font color="#FFFFFF">'''Adverse Event'''</font></center> | ||
| + | |align = "center" bgcolor = "#4f81bd"|<center><font color="#FFFFFF">'''Date FDA Received'''</font></center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<center>'''1'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.cfm?MDRFOI__ID=660847 Cook Urologicals Cook Urological Stent]</u></font> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|Stent broke into pieces while removing it from the patients body. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|12/14/2005 | ||
| + | |- | ||
| + | | style="padding:0.079cm;"|<center>'''2'''</center> | ||
| + | | style="padding:0.079cm;"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=930422 Boston Scoientific Boston Scientific Ureteral stent System]</u></font> | ||
| + | | style="padding:0.079cm;"|Fractured stent seen under Fluroscopy | ||
| + | | style="padding:0.079cm;"|10/17/2007 | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<center>'''3'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=755260 Boston Scoientific Boston Scientific Ureteral Stent System Kit 8 FR X 24 CM]</u></font> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|During insertion of ureteral stent, the stent broke into multiple parts which were retained in the patient. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|10/14/2005 | ||
| + | |- | ||
| + | | style="padding:0.079cm;"|<center>'''4'''</center> | ||
| + | | style="padding:0.079cm;"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=564910 Boston Scientific Corp Boston Scientific 8 FR X 28 CM Ureteral Stent System Kit]</u></font> | ||
| + | | style="padding:0.079cm;"|Breakage of the upper loop of the ureteral stent while trying to insert it. | ||
| + | | style="padding:0.079cm;"|01/05/2005 | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<center>'''5'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=522129 Boston Scientific Bostoon Scientific Micro Vasive Contour VL Ureteral Stent]</u></font> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|Broken stent observed during x-ray procedure. | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"|12/12/2003 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==Pre-Market Notification== | ||
| + | Section 510(k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical device at least 90 days in advance, also known as Premarket Notification. This premarket submission demonstrates to the FDA that the device to be marketed is atleast as safe and effective, that is, ''substantially equivalent'', to a legally marketed device. Parties required to submit a 510(k) to the FDA include domestic or foreign manufacturers introducing a device to the U.S. market, as well as specification developers and repackers/relabelers. | ||
| + | |||
| + | A 510(k) is required when: | ||
| + | * Introducing a device into commercial distribution (marketing) for the first time. | ||
| + | * Proposed different intended use for a device already in commercial distribution. | ||
| + | * Change or modification of a legally marketed device. | ||
| + | |||
| + | [http://dolcera.com/upload/files/510kflowchart.pdf 510(k) “Substantial Equivalence” Decision Making Process] | ||
| + | |||
| + | Some of the companies active in the field of ureteral stents have been represented in the table below. | ||
| + | |||
| + | {| border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''S.No. '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"|<center><font color="#FFFFFF">'''Company '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Device '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Approval '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Date of Approval '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Material '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Technology '''</font></center> | ||
| + | | style="background-color:#4f81bd;padding:0.079cm;"| <center><font color="#FFFFFF">'''Indwelling time (days) '''</font></center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>'''1'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.bardurological.com/products/categoryTwo.aspx?bUnitID=3&catOneID=71 Bard Urological]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[[Image:InLay_Optima.png|thumb|center|100px|<center>InLay Optima</center>|[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3∏ID=225 <center>InLay Optima</center>]]] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=16869 FDA 510(k)]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>Dec 2004</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Silicone | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Double pigtail with monofilament suture loop | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>365</center> | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>'''2'''</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Boston Scientific]</center> | ||
| + | | style="padding:0.079cm;"| [[Image:Polaris_Loop.png|thumb|center|100px|[http://www.bostonscientific.com/urology-stone/product.html?method=product_detail∏uct_id=10122561#initialLoad1() <center>Polaris Loop</center>]]] | ||
| + | | style="padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=10929 FDA 510(k)]</center> | ||
| + | | style="padding:0.079cm;"| <center>Mar 2003</center> | ||
| + | | style="padding:0.079cm;"| Dual Durometer Percuflex with HydroPlus Coating | ||
| + | | style="padding:0.079cm;"| Bladder loop design | ||
| + | | style="padding:0.079cm;"| <center>365</center> | ||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>'''3'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Cook Medical]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [[Image:Resonance.png|thumb|center|100px|[http://www.cookmedical.com/uro/dataSheet.do?id=4418 <center>Resonance</center>]]] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=23620 FDA 510(k)]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>May 2007</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Metal | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Temporary stenting | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>365</center> | ||
| + | |- | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>'''4'''</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>[http://www.fossamedical.com/news.htm Fossa Medical]</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| [[Image:Stone_Sweeper.png|thumb|center|100px|[http://dolcera.com/upload/files/stonesweeper_fossa_trial.pdf <center>Stone Sweeper</center>]]] | ||
| + | | style="padding:0.079cm;"| <center>[http://www.fossamedical.com/news.htm FDA 510(k)]</center> | ||
| + | | style="padding:0.079cm;"| <center>Aug 2002</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| Polyurethane | ||
| + | | style="padding:0.079cm;" rowspan="2"| Spiral radially expanding stent | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>13</center> | ||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>[http://www.fossamedical.com/news.htm CE Mark]</center> | ||
| + | | style="padding:0.079cm;"| <center>Sep 2005</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>'''5'''</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.pnnmedical.com/urology/professionals/products/memokath™-051-ureter.aspx Pnn Medical A/S]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| [[Image:Memokath_051.png|thumb|center|100px|[http://www.google.com/url?sa=t&source=web&cd=4&ved=0CC4QFjAD&url=http://www.hammer.pl/pliki/147_2.pdf&rct=j&q=memokath%20051&ei=MfhATezNIoaqvQP-_ZGtAw&usg=AFQjCNFR-ZFsu33rk6B9Flq1tCsYBZyXMw&cad=rja <center>Memokath 051</center>]]] | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>[http://www.pnnmedical.com/about-pnn-medical/company-history.aspx CE Mark]</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>1995</center> | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Nickel-titanium shape memory alloy | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| Double fluted ended spiral stent | ||
| + | | style="background-color:#dce6f1;padding:0.079cm;"| <center>240</center> | ||
| + | |} | ||
| + | |||
| + | ==Timeline Visualization == | ||
| + | [[Media:Ureteral_Stents_Timeline dw.xls|Ureteral Stent Timeline]] | ||
| + | |||
| + | =Products= | ||
| + | The FDA classifies a ureteric stent as follows: | ||
| + | * TITLE 21 - FOOD AND DRUGS | ||
| + | * CHAPTER I - FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES | ||
| + | * SUBCHAPTER H - MEDICAL DEVICES | ||
| + | * PART 876 - GASTROENTEROLOGY-UROLOGY DEVICES | ||
| + | * Subpart E - Surgical Devices | ||
| + | * Sec. 876.4620 - Ureteral stent. | ||
| + | * Classification - class II device [http://www.accessdata.fda.gov/SCRIPTS/cdrh/cfdocs/cfCFR/CFR.cfm?fr=876.4620&Term=ureter%20stent Code of Federal Regulations] | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Sr. No.''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Company''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Device(s)''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Approval''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Approval Date''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Material''' | ||
| + | |bgcolor = "#4f81bd"|'''Technology''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Indwelling Time (days)''' | ||
| + | |align = "center" bgcolor = "#4f81bd"|'''Image''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|'''1''' | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.allium-medical.com/?categoryId=64772 Allium, Israel]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|[http://www.allium-medical.com/?categoryId=64772 URS] | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF">[http://www.highbeam.com/doc/1G1-165990208.html CE Mark]</font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Jul, 2007 | ||
| + | |bgcolor = "#dce6f1"|Nickel-titanium shape memory alloy covered by polymer | ||
| + | |bgcolor = "#dce6f1"|Self-expanding stent | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|[[Image:Allium.png|thumb|center|100px|<center>Allium</center>]] | ||
| + | |||
| + | |- | ||
| + | |align = "center" rowspan = "2"|'''2''' | ||
| + | |align = "center" rowspan = "2"|<font color="#0000FF">[http://www.pnnmedical.com/urology/professionals/products/memokath™-051-ureter.aspx Pnn Medical A/S]</font> | ||
| + | |align = "center" rowspan = "2"|[http://www.google.com/url?sa=t&source=web&cd=4&ved=0CC4QFjAD&url=http://www.hammer.pl/pliki/147_2.pdf&rct=j&q=memokath%20051&ei=MfhATezNIoaqvQP-_ZGtAw&usg=AFQjCNFR-ZFsu33rk6B9Flq1tCsYBZyXMw&cad=rja Memokath 051] | ||
| + | |align = "center"|<font color="#0000FF">[http://www.pnnmedical.com/about-pnn-medical/company-history.aspx CE Mark]</font> | ||
| + | |align = "center"|1995 | ||
| + | |rowspan = "2"|Nickel-titanium shape memory alloy | ||
| + | |rowspan = "2"|Double fluted ended spiral stent | ||
| + | |align = "center" rowspan = "2"|240 | ||
| + | |rowspan = "2"|[[Image:Memokath_051.png|thumb|center|100px|<center>Memokath 051</center>]] | ||
| + | |||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.zapconnect.com/products/index.cfm/fuseaction/products_display_detail/eregnum/8021561/owner_operator_number/8021561/product_code/FAD/8021561.html FDA Listing]</u></font> | ||
| + | |align = "center"|Mar, 2004 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "5"|'''3''' | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "5"|<font color="#0000FF"><u>[http://www.fossamedical.com/news.htm Fossa Medical]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "2"|[http://dolcera.com/upload/files/stonesweeper_fossa_trial.pdf Stone Sweeper] | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.fossamedical.com/news.htm CE Mark]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Sep, 2005 | ||
| + | |bgcolor = "#dce6f1" rowspan = "2"|Polyurethane | ||
| + | |bgcolor = "#dce6f1" rowspan = "2"|Radially expanding stent | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "2"|13 | ||
| + | |rowspan = "2" bgcolor = "#dce6f1"|[[Image:Stone_Sweeper.png|thumb|center|100px|<center>Stone Sweeper</center>]] | ||
| + | |||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.fossamedical.com/news.htm FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Aug, 2002 | ||
| + | |||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "2"|[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/PMNSimple.cfm?db=PMN&ID=K033368 Open lumen stent] | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/PMNSimple.cfm?db=PMN&ID=K033368 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Nov, 2003 | ||
| + | |bgcolor = "#dce6f1" rowspan = "2"|Polyurethane | ||
| + | |bgcolor = "#dce6f1" rowspan = "2"|Pigtail-tipped stent with <nowiki>’</nowiki>Pusher<nowiki>’</nowiki> | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "2"| | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "2"| [[Image:Open_Lumen.png|thumb|center|100px|<center>Open_lumen</center>]] | ||
| + | |||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.fossamedical.com/news.htm CE Mark]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Sep, 2005 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/PMNSimple.cfm?db=PMN&ID=K021140 Expanding Ureteral Stent] | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/PMNSimple.cfm?db=PMN&ID=K021140 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Jun, 2002 | ||
| + | |bgcolor = "#dce6f1"|Polyurethane | ||
| + | |bgcolor = "#dce6f1"|Double pigtail stent with <nowiki>’</nowiki>Pusher<nowiki>’</nowiki> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| N/A | ||
| + | |||
| + | |- | ||
| + | |align = "center" rowspan = "7"|'''4''' | ||
| + | |align = "center" rowspan = "7"|<font color="#0000FF"><u>[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Boston Scientific]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>Contour</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Percuflex - proprietary polyolefin copolymer; Hydroplus coating | ||
| + | |Fixed and variable length; Tapered tip | ||
| + | |align = "center"|365 | ||
| + | |align = "center" |[[Image:Contour.png|thumb|center|100px|<center>Contour</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>Percuflex</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |align = "center"| Percuflex | ||
| + | |Pigtail | ||
| + | |align = "center"|365 | ||
| + | |align = "center" |[[Image:Percuflex.png|thumb|center|100px|<center>Percuflex</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>Polaris Ultra</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/PMNSimple.cfm?db=PMN&id=K010002 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Jan, 2001 | ||
| + | |Dual Durometer Percuflex with HydroPlus Coating; soft Nautilus Bladder Coil. | ||
| + | |Double pigtail | ||
| + | |align = "center"|365 | ||
| + | |align = "center" |[[Image:Polaris_Ultra.png|thumb|center|100px|<center>Polaris Ultra</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>Polaris Loop</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=10929 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Mar, 2003 | ||
| + | |Dual Durometer Percuflex with HydroPlus Coating | ||
| + | |Bladder loop design | ||
| + | |align = "center"|365 | ||
| + | |align = "center" |[[Image:Polaris_Loop.png|thumb|center|100px|<center>Polaris Loop</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.google.com/url?sa=t&source=web&cd=4&ved=0CCkQFjAD&url=http://www.bostonscientific.com/templatedata/imports/Microsite/Stone-EU/collateral/stone-eu-percuflex-brochure-eng.pdf&rct=j&q=percuflex%20brochure&ei=bIE-Tb79HMnqrAf5-_HRCA&usg=AFQjCNEJ-JOc Retromax Plus]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Percuflex material and Hydroplus coating | ||
| + | |Endopyelotomy stent | ||
| + | |align = "center"|Post-procedure healing | ||
| + | |align = "center" |[[Image:Retromax_plus.png|thumb|center|100px|<center>Retromax Plus</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.google.com/url?sa=t&source=web&cd=4&ved=0CCkQFjAD&url=http://www.bostonscientific.com/templatedata/imports/Microsite/Stone-EU/collateral/stone-eu-percuflex-brochure-eng.pdf&rct=j&q=percuflex%20brochure&ei=bIE-Tb79HMnqrAf5-_HRCA&usg=AFQjCNEJ-JOc Stretch VL Flexima]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Hydroplus Coating | ||
| + | |Variable length coil on distal and proximal ends | ||
| + | |align = "center"|90 | ||
| + | |align = "center" |[[Image:Stretch_VL_Flexima.png|thumb|center|100px|<center>Stretch VL Flexima</center>]] | ||
| + | |- | ||
| + | |align = "center"|[http://dolcera.com/upload/files/drug_eluting_ureteral_stent.pdf Drug-Eluting Stent] | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Percuflex - proprietary polyolefin copolymer | ||
| + | |Ketorolac trimethamine loaded stent | ||
| + | |align = "center"| | ||
| + | |align = "center"| N/A | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "6"|'''5''' | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "6"|<font color="#0000FF">[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Cook Medical]</font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.cookmedical.com/uro/dataSheet.do?id=4418 Resonance]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=23620 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|May, 2007 | ||
| + | |bgcolor = "#dce6f1"|Metal | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|365 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Resonance.png|thumb|center|100px|<center>Resonance</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.cookmedical.com/uro/dataSheet.do?id=2055 Sof-flex]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|AQ® Hydrophilic Coating | ||
| + | |bgcolor = "#dce6f1"|Radiopaque tip and tether for repositioning | ||
| + | |align = "center" bgcolor = "#dce6f1"|180 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Sof_flex.png|thumb|center|100px|<center>Sof-flex</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.cookmedical.com/uro/dataSheet.do?id=3627 Endo-Sof]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|AQ® Hydrophilic Coating | ||
| + | |bgcolor = "#dce6f1"|Double pigtail | ||
| + | |align = "center" bgcolor = "#dce6f1"|365 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Endo_Sof.png|thumb|center|100px|<center>Endo-Sof</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.cookmedical.com/uro/dataSheet.do?id=3643 C-Flex]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|Double Pigtail | ||
| + | |align = "center" bgcolor = "#dce6f1"|180 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:C_Flex.png|thumb|center|100px|<center>C-Flex</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.cookmedical.com/uro/dataSheet.do?id=4692 Smith Universal]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|Nephrostomy tube <nowiki>+</nowiki> Ureteral stent | ||
| + | |align = "center" bgcolor = "#dce6f1"|60 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Smith_Universal.png|thumb|center|100px|<center>Smith Universal</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.medicalnewstoday.com/articles/90717.php Endo-Sof Radiance]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.medicalnewstoday.com/articles/90717.php Launch]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Dec, 2007 | ||
| + | |bgcolor = "#dce6f1"|Heparin-bonded stent | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |- | ||
| + | |align = "center"|'''6''' | ||
| + | |align = "center"|<font color="#0000FF">[http://qurological.com/product/ Q Urological]</font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://qurological.com/product/ pAguaMedicina™ Pediatric Ureteral Stent]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=29056 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Jan, 2010 | ||
| + | |Hydrogel | ||
| + | |Differentially larger end (no pigtail) | ||
| + | |align = "center"|30 | ||
| + | |align = "center" |[[Image:pAguaMedicina.png|thumb|center|100px|<center>pAguaMedicina</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|'''7''' | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF">[http://www.bioteq.com.tw/en/products.php?kind=2&series=4 Bioteque Corp.]</font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Ureteral Stent Set | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bioteq.com.tw/en/news_detail.php?id=1&query_string= FDA 510(k) ]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Apr, 2010 | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|30 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:bioteque.png|thumb|center|100px|<center>Bioteque</center>]] | ||
| + | |- | ||
| + | |align = "center" rowspan = "4"|'''8''' | ||
| + | |align = "center" rowspan = "4"|<font color="#0000FF">[http://www.appliedmed.com/products/product_card.aspx?section=professionals&proceGroupID=4&groupName=Urology&catID=37&Name=Ureteral+stents Applied Medical Resources, CA, USA]</font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=4353 Mesh]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=4353 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Jul, 2001 | ||
| + | |Polyester mesh | ||
| + | |Double-pigtail | ||
| + | |align = "center"| | ||
| + | |align = "center"| N/A | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.appliedmed.com/products/product_card.aspx?section=professionals&proceGroupID=4&groupName=Urology&catID=37&Name=Ureteral+stents Silhouette ]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Coil-reinforced; SL-6® hydrophilic coating | ||
| + | |Patency Device | ||
| + | |align = "center"| | ||
| + | |align = "center" |[[Image:silhouette.png|thumb|center|100px|<center>Silhouette</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.appliedmed.com/products/product_card.aspx?section=professionals&proceGroupID=4&groupName=Urology&catID=37&Name=Ureteral+stents Applied Standard]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=124645 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Jun, 1999 | ||
| + | |Proprietary thermoplastic elastomer material; SL-6® hydrophilic coating | ||
| + | |Unique wall construction and enlarged drainage holes | ||
| + | |align = "center"| | ||
| + | |align = "center" |[[Image:Applied_Std1.png|thumb|center|100px|<center>Applied Standard</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.appliedmed.com/products/product_card.aspx?section=professionals&proceGroupID=4&groupName=Urology&catID=37&Name=Ureteral+stents 7-10 endopyelotomy]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Proprietary thermoplastic elastomer material; SL-6® hydrophilic coating | ||
| + | |Dual Diameter stent | ||
| + | |align = "center"| | ||
| + | |align = "center" |[[Image:Applied_7_10.png|thumb|center|100px|<center>Endopyelotomy Stent</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "6"|'''9''' | ||
| + | |align = "center" bgcolor = "#dce6f1" rowspan = "6"|<font color="#0000FF"><u>[http://www.bardurological.com/products/categoryTwo.aspx?bUnitID=3&catOneID=71 Bard Urological]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=225 InLay Optima]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=16869 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Dec, 2004 | ||
| + | |bgcolor = "#dce6f1"|Silicone | ||
| + | |bgcolor = "#dce6f1"|Double pigtail with monofilament suture loop | ||
| + | |align = "center" bgcolor = "#dce6f1"|365 | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:InLay_Optima.png|thumb|center|100px|<center>InLay Optima</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=227 Bardex® Double Pigtail Soft Stent]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=8912 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Jan, 2003 | ||
| + | |bgcolor = "#dce6f1"|Silicone | ||
| + | |bgcolor = "#dce6f1"|Attached with suture for ease of removal | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Bardex.png|thumb|center|100px|<center>Bardex</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=228 Fluoro-4 Silicone Ureteral Stent]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|Silicone/tantalum | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Fluoro_4.png|thumb|center|100px|<center>Fluoro 4</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=230 Figure-4 Silicone Ureteral Stent]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|Silicone | ||
| + | |bgcolor = "#dce6f1"|Three dimensional design | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Figure_4.png|thumb|center|100px|<center>Figure 4</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=226 InLay Ureteral Stent]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=122796 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Dec, 1998 | ||
| + | |bgcolor = "#dce6f1"|Silicone | ||
| + | |bgcolor = "#dce6f1"|Tapered tip and lubricious hydrophilic coating | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:InLay.png|thumb|center|100px|<center>InLay</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3&prodID=229 Urinary Diversion Stent]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=86128 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Apr, 1991 | ||
| + | |bgcolor = "#dce6f1"|Silicone | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Urinary_Diversion_Stent.png|thumb|center|100px|<center>Urinary Diversion Stent</center>]] | ||
| + | |- | ||
| + | |align = "center" rowspan = "4"|'''10''' | ||
| + | |align = "center" rowspan = "4"|<font color="#0000FF">[http://www.coloplast.com/Pages/home.aspx Coloplast-Porges]</font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.porges.ru/catalog.html?cid=251 Vortek]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=121312 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Oct, 1998 | ||
| + | |Silicone | ||
| + | |Double coating for easy maneuverability as well as flexibility | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Vortek.png|thumb|center|100px|<center>Vortek</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.porges.ru/catalog.html?cid=248 Biosoft]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=121312 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Oct, 1998 | ||
| + | |Silicone | ||
| + | |Extreme flexibility | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Biosoft.png|thumb|center|100px|<center>Biosoft</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.porges.ru/catalog.html?cid=249 Polyurethane]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Hard or soft Polyurethane | ||
| + | |Designed for short-term use | ||
| + | |align = "center"| 90 | ||
| + | |align = "center"|[[Image:Polyurethane1.png|thumb|center|100px|<center>Polyurethane</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.porges.ru/catalog.html?cid=250 Silicone]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=6586 FDA 510k)]</u></font> | ||
| + | |align = "center"|Oct, 2002 | ||
| + | |Silicone | ||
| + | |''Pyatiprofilnaya'' technology | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Silicone1.png|thumb|center|100px|<center>Silicone</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|'''11''' | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF">[http://www.teleflex.com/en/emea/productAreas/urology/index.html Teleflex Medical]</font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.google.com/url?sa=t&source=web&cd=3&ved=0CCQQFjAC&url=http%3A%2F%2Fwww.myrusch.com%2Fimages%2Frusch%2Fdocs%2FU62C.pdf&rct=j&q=DD%2Bureteral%2Bstent&ei=CcY-TeDWHcrirAfyr4naCA&usg=AFQjCNHSSc9r_DBwotSa_oszWLYMPRuoYg&cad=rja Rüsch Superglide DD]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=122381 FDA 510(k)]</u></font> | ||
| + | |align = "center" bgcolor = "#dce6f1"|Jul, 1999 | ||
| + | |bgcolor = "#dce6f1"| WIRUTHAN® (polyurethane) with hydrogel coating | ||
| + | |bgcolor = "#dce6f1"| Directable and detachable | ||
| + | |align = "center" bgcolor = "#dce6f1"| | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Superglide_DD.png|thumb|center|100px|<center>Superglide DD</center>]] | ||
| + | |- | ||
| + | |align = "center" rowspan = "9"|'''12''' | ||
| + | |align = "center" rowspan = "9"|<font color="#0000FF">[http://www.gyrusacmi.com/user/display.cfm?display=cat_menu&maincat=Stone%20Management&catid=122 Gyrus ACMI/Cabot/Acromed/Circon/Surgitek]</font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9257&catid=122&maincat=Stone%20Management&catname=Stents Classic closed-tip]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=68160 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Dec, 1986 | ||
| + | | | ||
| + | |Classic Closed Tip | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Gyrus_Closed_Tip.png|thumb|center|100px|<center>Classic Closed Tip</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9252&catid=122&maincat=Stone%20Management&catname=Stents Classic Double pigtail]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=111987 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Mar, 1996 | ||
| + | |Tecoflex® construction | ||
| + | |Balanced-curled double pigtail design | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Gyrus_Pigtail.png|thumb|center|100px|<center>Double Pigtail</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9256&catid=122&maincat=Stone%20Management&catname=Stents Double-J]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=74392 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Apr, 1988 | ||
| + | |Silicone | ||
| + | |Double-J closed-tip | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Gyrus_Double_J.png|thumb|center|100px|<center>Double_J</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9254&catid=122&maincat=Stone%20Management&catname=Stents Lithostent]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Tecoflex® | ||
| + | |Grooved design | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Lithostent.png|thumb|center|100px|<center>Lithostent</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9253&catid=122&maincat=Stone%20Management&catname=Stents Lubri-flex]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=91169 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Nov, 1991 | ||
| + | |Tecoflex® | ||
| + | |“Rememberance” of shape with a chemically bonded wettable solution | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Lubri_Flex.png|thumb|center|100px|<center>Lubri-flex</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9250&catid=122&maincat=Stone%20Management&catname=Stents Multi-flex]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Tecoflex® | ||
| + | |Two durometers with helical kidney curls | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Multi_Flex.png|thumb|center|100px|<center>Multi-flex</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9255&catid=122&maincat=Stone%20Management&catname=Stents Quadra-Coil multi-length]</u></font> | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=107077 FDA 510(k)]</u></font> | ||
| + | |align = "center"|Mar, 1996 | ||
| + | |Tecoflex® | ||
| + | |Accomodate ureteral lengths from 22cm to 28cm | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Quadra_Coil.png|thumb|center|100px|<center>Quadra-Coil</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9249&catid=122&maincat=Stone%20Management&catname=Stents Sof-curl]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Tecoflex® | ||
| + | |Dual-durometer design and exclusive soft bladder helix | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Sof_curl.png|thumb|center|100px|<center>Sof-curl</center>]] | ||
| + | |- | ||
| + | |align = "center"|<font color="#0000FF"><u>[http://www.gyrusacmi.com/user/display.cfm?display=product&pid=9258&catid=122&maincat=Stone%20Management&catname=Stents Uroguide]</u></font> | ||
| + | |align = "center"| | ||
| + | |align = "center"| | ||
| + | |Silicone | ||
| + | |Classic Double J with open tip | ||
| + | |align = "center"| | ||
| + | |align = "center"|[[Image:Uroguide.png|thumb|center|100px|<center>Uroguide</center>]] | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#dce6f1"|'''13''' | ||
| + | |align = "center" bgcolor = "#dce6f1"|[http://www.amecath.com/ Ameco Medical Industries] | ||
| + | |align = "center" bgcolor = "#dce6f1"|[http://www.amecath.com/ Amecath] | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"| | ||
| + | |bgcolor = "#dce6f1"|Nitinol; Available with hydrophilic coating | ||
| + | |bgcolor = "#dce6f1"|Double loop stent | ||
| + | |align = "center" bgcolor = "#dce6f1"|Short-term and long-term | ||
| + | |align = "center" bgcolor = "#dce6f1"|[[Image:Amecath.png|thumb|center|100px|<center>Amecath</center>]] | ||
| + | |- | ||
| + | |align = "center"|'''14''' | ||
| + | |align = "center"|[http://www.zapconnect.com/companies/index.cfm/fuseaction/companies_detail/eregnum/9681442.html Angiomed-Movaco (C.R. Bard subsidiary)] | ||
| + | |align = "center"|[http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K864225 Ureteral Stent Set] | ||
| + | |align = "center"|[http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K864225 FDA 510(k)] | ||
| + | |align = "center"|Jan, 1987 | ||
| + | |Nitinol | ||
| + | |Self-expanding stent | ||
| + | | | ||
| + | |align = "center"|N/A | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | =Patent-Product-Clinical Trial Mapping= | ||
| + | * To access the Dashboard you have to signup. You can do so by clicking [https://www.dolcera.com/auth/index.php/login '''here'''] | ||
| + | *''Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy'' | ||
| + | *''Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard'' | ||
| + | *''Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap'' | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |||
| + | |<mm>[[ureteral_stent_mapping1.mm|flash|Ureteral Stent mindmap|600pt]]</mm> | ||
| + | |||
| + | |} | ||
| + | |||
| + | = Product to Patent Mapping = | ||
| + | [[Image:Product_Patent_Mapping_Screen_Shot.png|1000px|centre|thumb|'''Screenshot for the product to patent mapping(Bard and Boston)''']] | ||
| + | * Click [[Media:Product_Patent_Mapping_Bard_Boston.xls|'''here ''']]to download the excel file. | ||
| + | * Mapped Patent vs Not Mapped Patents | ||
| + | {|border="0" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |[[Image:CRB_Pat.png|center|500px|thumb|'''C R Bard''']] | ||
| + | |[[Image:BS_pat.png.png|center|500px|thumb|'''Boston Scientific''']] | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | =Key Findings= | ||
| + | ==Major Players== | ||
| + | *Boston Scientific Limited, Abbott, Medtronic and Cook Inc. are the major players in ureteral stent research field. | ||
| + | [[Image:Major playersnew.jpg|thumb|center|1000px|Major Players]] | ||
| + | |||
| + | ==Key Patents== | ||
| + | *The key patents in the field are held by Cook Incorporated, Interventional Thermodynamics, Abbott laboratories and Boston Scientific Corp. | ||
| + | [[Image:Key patent2.png|thumb|center|1100px|Key Patents]] | ||
| + | |||
| + | ==IP Activity== | ||
| + | *Patenting activity has been high growth rate during the period 2001 to 2005 with a peak no. of patents in year 2005, followed by saturation during the period 2006 to 2008 and after that a gradual declination upto year 2010 in the ureteral stent research area. | ||
| + | [[Image:IPactivity3.png|thumb|center|1000px|year wise IP activity]] | ||
| + | |||
| + | ==Geographical Activity== | ||
| + | *USA, Europe and Germany are very active in ureteral stent research. | ||
| + | [[Image:Geodistribution5.png|thumb|center|600px|Geographical Activity]] | ||
| + | |||
| + | |||
| + | |||
| + | *References | ||
| + | |||
| + | 1- [http://en.wikipedia.org/wiki/Demographics_of_the_United_States http://en.wikipedia.org/wiki/Demographics_of_the_United_States] | ||
| + | |||
| + | 2- [http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink] | ||
| + | |||
| + | 3- [http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf] | ||
| + | |||
| + | 4- [http://www.managementparadise.com/forums/principles-management-p-o-m/208329-swot-analysis-boston-scientific-corporation.html http://www.managementparadise.com/forums/principles-management-p-o-m/208329-swot-analysis-boston-scientific-corporation.html] | ||
| + | |||
| + | ==Trends in stent R&D== | ||
| + | |||
| + | * Given that the first bare metal stent entered the market in 1994, the technology is far from mature. | ||
| + | |||
| + | * Whilst drug eluting stents will be improved through the use of what are described as more ‘cell friendly’ (i.e. more hydrophobic) coatings and adapted (by using CFD in stent design) to varying wall sheer conditions, the industry is already well down the road of developing bioresorbable coatings and even bioresorbable stents. | ||
| + | |||
| + | * Such systems are already on limited trial in Europe with FDA trials aimed at 2012 and approval by 2015. | ||
| + | |||
| + | * There are still other areas under study including thinner struts which are important in terms of giving improved stent flexibility, a reduced cross-sectional profile within the vessel and potentially reduced restenosis (due to reduced vascular trauma). | ||
| + | |||
| + | * Silicon carbides, carbon coatings, titanium nitride/oxide and sputtered indium oxide have all been trialled as routes to reducing the incidence of restenosis and/or thrombosis. | ||
| + | |||
| + | * Click here for recent news and developments in [[Boston Scientific]] and [[Cook INC]] | ||
| + | |||
| + | '''Source''' [http://www.ceram.com/newsroom/news-releases/ceram-examines-the-future-of-stent-materials/ Ceram-newsrelease] | ||
| + | |||
| + | |||
| + | =Competitor Analysis= | ||
| + | |||
| + | Total Sales in 2010 - 4.04 Billion USD | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |bgcolor = "#5CB3FF"|'''Company''' | ||
| + | |bgcolor = "#5CB3FF"|'''Total Sales in 2010''' | ||
| + | |bgcolor = "#5CB3FF"|'''Urological sales''' | ||
| + | |bgcolor = "#5CB3FF"|'''Percentage share''' | ||
| + | |bgcolor = "#5CB3FF"|'''Product portfolio''' | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|'''Boston Scientific''' | ||
| + | |7800 | ||
| + | |661 | ||
| + | |8.48 | ||
| + | |<font color="#0000FF"><u>[http://www.bostonscientific.com/templatedata/imports/Microsite/Stone/collateral/Percuflex-Brochure.pdf Boston_portfolio]</u></font> | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|'''CR BARD''' | ||
| + | |2700 | ||
| + | |702 | ||
| + | |26.00 | ||
| + | |<font color="#0000FF"><u>[https://dolcera.net/teamwiki_prod/index.php/BARD_portfolio BARD_portfolio]</u></font> | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|'''Cook Medical''' | ||
| + | |1700 | ||
| + | |<nowiki>-</nowiki> | ||
| + | |<nowiki>-</nowiki> | ||
| + | |<font color="#0000FF"><u>[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Cook_portfolio]</u></font> | ||
| + | |- | ||
| + | |bgcolor = "#5CB3FF"|'''Medline''' | ||
| + | |4040 | ||
| + | |<nowiki>-</nowiki> | ||
| + | |<nowiki>-</nowiki> | ||
| + | |<font color="#0000FF"><u>[http://www.medline.com/irj/catalog/search?initialSearchTerms=ureteral%20stent Medline_portfolio]</u></font> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | All figures in USD million | ||
| + | |||
| + | [[image:stentshare.jpg]] | ||
| + | |||
| + | |||
| + | ====Boston Scientific==== | ||
| + | |||
| + | |||
| + | [https://dolcera.net/teamwiki_prod/index.php/Boston_Scientific_-_Company_Profile Company profile] | ||
| + | |||
| + | [http://www.bostonscientific.com/templatedata/imports/Microsite/Stone/collateral/Percuflex-Brochure.pdf Ureteral Stent portfolio] | ||
| + | |||
| + | Net sales in 2010 – USD 7.8 Billion | ||
| + | |||
| + | Share of Urology- 8.48 % | ||
| + | |||
| + | Net sales from Urology- USD 661 Million | ||
| + | |||
| + | Source [http://phx.corporate-ir.net/phoenix.zhtml?c=62272&p=irol-reportsother BSsalesdata] | ||
| + | |||
| + | ====CR BARD==== | ||
| + | |||
| + | [[Urology portfolio]] | ||
| + | |||
| + | |||
| + | Net sales in 2010 – USD 2.7 Billion | ||
| + | |||
| + | Share of Urology- 26% | ||
| + | |||
| + | Net sales from Urology- USD 702 Million | ||
| + | |||
| + | Source – CR BARD annual report | ||
| + | |||
| + | ====Cook Medical==== | ||
| + | |||
| + | [http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Ureteral Stent portfolio] | ||
| + | |||
| + | Total Sales - USD 1.7 Billion | ||
| + | |||
| + | [http://www.nytimes.com/2011/04/26/business/26cook.html?_r=1 Source] | ||
| + | |||
| + | ====Medline==== | ||
| + | |||
| + | [http://www.medline.com/irj/catalog/search?initialSearchTerms=ureteral%20stent Ureteral Stent Portfolio] | ||
| + | |||
| + | |||
| + | ==Academic Research == | ||
| + | |||
| + | Research at University of Minnesota about symptoms and pain associated with ureteral stent | ||
| + | |||
| + | [[Research Team]] | ||
| + | |||
| + | '''Background and Purpose''' | ||
| + | |||
| + | *Ureteral stents are associated with significant pain and urinary symptoms. | ||
| + | |||
| + | *Manufacturers have altered stent designs and materials in an attempt to minimize this morbidity. | ||
| + | |||
| + | *This study evaluated the impact of these modifications. | ||
| + | |||
| + | '''Patients and Methods''' | ||
| + | |||
| + | *Stent manufacturers were asked to provide the 6F ureteral stent they believed would be associated with the least patient discomfort. | ||
| + | |||
| + | *Patients undergoing uncomplicated ureteroscopy were randomized to the Bard Inlay, Cook Endo-Sof, Microvasive Contour, Applied Medical Vertex, or Surgitek Classic Double-Pigtail stent. | ||
| + | |||
| + | *The Ureteric Stent Symptom Questionnaire (USSQ) was administered on days 1, 3, and 5, and the patients maintained a narcotic diary. The data were analyzed using ANOVA and nonparametric methods. | ||
| + | |||
| + | '''Results''' | ||
| + | |||
| + | *A total of 44 patients (73%) completed all USSQ questionnaires. | ||
| + | |||
| + | *Urinary symptom scores were significantly lower for the Inlay stent on day 3 than for the Vertex (P = 0.01), Contour (P = 0.05), Endo-Sof (P = 0.03), and Classic (P = 0.02) stents. No significant differences were noted in pain and general symptom scores or narcotic use. | ||
| + | |||
| + | '''Conclusion''' | ||
| + | |||
| + | *'''The Bard Inlay stent is associated with less-severe urinary symptoms than other ureteral stents.''' | ||
| + | |||
| + | *The USSQ is a sensitive tool to measure differences between stents. | ||
| + | |||
| + | The complete USSQ form is available at [http://www.bui.ac.uk/ BUI] and [http://www.endourology.org/ endourology]. | ||
| + | |||
| + | Source [http://www.liebertonline.com/doi/abs/10.1089/end.2005.19.990?journalCode=end University of Minnesota] | ||
| + | |||
| + | =Removed Sections= | ||
| + | [[Removed Sections]] | ||
| + | |||
| + | =<span style="color:#C41E3A">Like this report?</span>= | ||
| + | <p align="center"> '''This is only a sample report with brief analysis''' <br> | ||
| + | '''Dolcera can provide a comprehensive report customized to your needs'''</p> | ||
| + | {|border="2" cellspacing="0" cellpadding="4" align="center" " | ||
| + | |style="background:lightgrey" align = "center" colspan = "3"|'''[mailto:info@dolcera.com <span style="color:#0047AB">Buy the customized report from Dolcera</span>]''' | ||
| + | |- | ||
| + | | align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services Patent Analytics Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/business-research-services Market Research Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools/patent-dashboard Purchase Patent Dashboard] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Landscape Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/research-processes Dolcera Processes] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/industries Industry Focus] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Search Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/alerts-and-updates Patent Alerting Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools Dolcera Tools] | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| + | |||
== Addition == | == Addition == | ||
=== Patent Categorization: Interactive mind map linked to Dolcera Dashboard === | === Patent Categorization: Interactive mind map linked to Dolcera Dashboard === | ||
Revision as of 06:01, 30 June 2011
This is a landscape report on the Ureteral stent market, including key company profiles, products, patents and relevant clinical trails.
- What is it? A ureteral stent is a specially designed hollow tube, made of a flexible plastic material that is placed in the ureter.
- Need for a ureteral stent: In patients who have, or might have, an obstruction (blockage) of the kidney, an internal drainage tube called a ‘stent’ is commonly placed in the ureter, the tube between the kidney and the bladder. This is placed there in order to prevent or temporarily relieve the obstruction.
Contents
Background
Ureteral stents are used in urological surgery to maintain patency of the ureter to allow urine drainage from the renal pelvis to the bladder. These devices can be placed by a number of different endourological techniques. They are typically inserted through a cystoscope and may also be inserted intraoperatively. Indwelling ureteral stents help to reduce complications and morbidity subsequent to urological and surgical procedures. Frequently, ureteral stents are used to facilitate drainage in conjunction with Extracorporeal Shock Wave Lithotripsy (ESWL) and after endoscopic procedures. They are also used to internally support anastomoses and prevent urine leakage after surgery. Ureteral stenting may almost eliminate the urological complications of renal transplantation. An antimicrobial ureteral stent, which inhibits encrustation and bacterial colonization while maintaining patient comfort.
- Ureteral stent: resists migration, resists fragmentation, is kink resistant and radiopaque.
- Bacterial colonization: antimicrobial activity for up to two weeks.
- Patient Comfort: stent has a low coefficient of friction (value) for ease of insertion and will soften on implant at body temperature to maintain patient comfort.
Market Overview
Market for ureteral stent can be analyzed by estimating market for each of Ureteral Stent’s fundamental use. Other uses of Ureteral Stent include Post-surgical swelling/infection of uterus, Active kidney infection etc.
Methodology
Kidney Stones Market
There are four main types of treatments available for curing kidney stones based on type of Kidney Stone, listed as follows
| Sr. No. | Treatment | Indications | Requirement of Ureteral Stent | % of cases in general |
| 1 | Lithotripsy | Radiolucent calculi, Renal stones <2 cm, Ureteral stones <1 cm | No | 49.07%* |
| 2 | Ureteroscopy | Ureteral stones | Yes | 15.48%* |
| 3 | Ureterorenoscopy | Renal stones <2 cm | Yes | 0.47%* |
| 4 | Percutaneous nephrolithotomy | Renal stones >2 cm, Proximal ureteral stones >1 cm | No | 34.98%* |
*Dolcera Estimate 2011
As seen in table ureteral stent is required for Ureteroscopy and Ureterorenoscopy which together constitute for around 16% of Kidney stone cases.
Total Number of Kidney Stone Cases in US in 2006: 166,000
Approximate Stents required in 2006 = 166,000*0.16=26560
Kidney Transplant Market
Every kidney transplant operation required ureteral stent to be placed in Patient’s body for few days till newly placed kidney adapts to Patients’ body
Total No. of Kidney Transplants done in United States in 2007 are approx. 17,513
Total Market for Ureteral Stents in 2007 is 17,513
| Year | No. of Kidney Transplants |
| 2007 | 17,513 |
| 2006 | 18,056 |
| 2005 | 17,443 |
| 2000 | 14,611 |
| 1995 | 12,160 |
| 1990 | 10,029 |
| 1985 | 7,504 |
| 1980 | 3,785 |
Urinary Incontinence Market
Inpatient hospital stays:The estimated number of hospital admissions among adults ages 18 or older with urinary incontinence listed as a diagnosis:
(2000): 47,802 hospital stays (1,332 men; 46,470 women)
| Prevalence of Urinary Incontinece in US (Women) | |
| Age (in years) | Population with Urinary Incontinence (in %) |
| 30-39 | 28 |
| 40-49 | 41 |
| 50-59 | 48 |
| 60-69 | 51 |
| 70-79 | 55 |
| ≥ 80 | 54 |
| Estimated cases for ureteral stents in US, 2010 | |||||
| Age groups | A) Female population (Million) | B) Prevalence rate in female (%) | C=A*B Affected population (Million) |
D) Catherization rate (%) | E=C*D Stent market based on catherization |
| 30-39 | 20.10 | 28 | 5.62 | 0.043 | 2420.42 |
| 40-49 | 21.99 | 41 | 9.01 | 0.123 | 11092.83 |
| 50-59 | 21.50 | 48 | 10.32 | 0.124 | 12800.37 |
| 60-69 | 15.32 | 51 | 7.81 | 0.160 | 12503.68 |
| 70-79 | 9.16 | 55 | 5.04 | 0.172 | 8674.44 |
| ≥ 80 | 7.15 | 54 | 3.86 | 0.044 | 1699.48 |
| Total | 95.25 | 41.69 | |||
- Catherization rate depicts the actual number of people going in for ureteral stents.
- Prevalence rate in US women is growing at a CAGR of 1.26%
Assuming that each admission in hospital required one ureteral stent, market from Urinary Incontinence is around 47,802 stents per year
Total Market for Ureteral Stent Following table displays approximate stent market per year
| Category | No. of Stents Required |
| Stent Market for Kidney Stones | 26560 |
| Stent Market for Kidney Transplant | 17513 |
| Stent Market for Urinary Incontinence | 47802 |
| Total | 91875 |
- Ureteral stent market is growing at a CAGR of 1.58%
Ureteral stent market forecast in US (women)
Interactive Mind Map
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
| Upload Taxonomy file Mmap905(1 (new1).1).mm |
| Upload Taxonomy file ureteral_stent_mapping.mm.mm |
Patents
Patent Search Strategy
Dolcera Patent Analysis
| |
|
|
|
|
| |
| |
| |||||
| 1 | US20090187254A1 | 07/23/2009 | BOSTON SCIENTIFIC SCIMED, INC. | UROLOGICAL MEDICAL DEVICES FOR RELEASE OF UROLOGICALLY BENEFICIAL AGENTS | In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | This invention provides a ueteral stent which release one or more urologically beneficial agents in effective amounts to reduce the problem of encrustation and pain related to stent. |
| 2 | US20090171465A1 | 07/02/2009 | Boston Scientific Scimed, Inc. | Polymeric Regions For Implantable Or Insertable Medical Devices | In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | This invention provides a ureteral stent with a coating of a material(EVA) that reduces the discomfort caused by the stent during implantation. |
| 3 | US20080097349A1 | 04/24/2008 | Boston Scientific Scimed, Inc. | Partially soluble implantable or insertable medical devices | In previous attempts to develope a uerteral stent, encrustation was the problem associated with the stent. Along with this pain and discomfort by stent implantaion was there. | Here the device (Stent) is provided with at least one surface that contains one or more depressions, which are at least partially filled with a soluble material. This makes stent more flexible and soft after implantation hence minimizing pain and discomfort after implantation or insertion. |
| 4 | US5554147A | 09/10/1996 | CApHCO, Inc. | Compositions and devices for controlled release of active ingredients | During implantation of ureteral stent,pain and discomfort are the problem with previous stents. Also microbial or bacterial infection at the site of implantation was the problem. | In this invention stent is povided with a pH sensitive coating that includes antimicrobial agent to stop the infection. This coating also has the hydrogel property which Upon swelling, the hydrogel's coefficient of friction is reduced, and the polymer becomes slippery, this mechanism reduces the discomfort. |
| 5 | US6468649B1 | 10/22/2002 | Scimed Life Systems, Inc. | Antimicrobial adhesion surface | In prior art problem was the lack of the device which can remain in vivo for extended periods of time without losing its antimicrobial efficacy and which can provides protection against bacterial and fungal organisms for extended periods of time without leaching substances into a patient. | This invention provides a medical device with a hydrophobic coating that inhibits the growth of microbes for extended period of time. |
| 6 | US20030153983A1 | 08/14/2003 | Scimed Life Systems, Inc. | Implantable or insertable medical device resistant to microbial growth and biofilm formation | In previous attempts to develope a ureteral stent with coating the problem was the formation of biofilm and microbial growth. | The device of the present invention, therefore, overcomes the disadvantages associated with the use of coatings as discussed above, and provides a reduced risk of biofilm fouling that eventually results in encrustation, occlusion and failure of the device. In this invention medical device comprises at least one biocompatible matrix polymer region and bioactive agents comprising an antimicrobial agent and a microbial attachment/biofilm synthesis inhibitor. |
| 7 | US20030139800A1 | 07/24/2003 | None | Stent assembly with therapeutic agent exterior banding | In earlier attempts for stent with a therapeutic property, the problem was the coating or the sheath (responsible for therapy) may covers side branch arteries, vessels, or other lumens extending from the main lumen in which the stent is installed. The sheath can reduce blood flow to or from the side branch and deliver medication into the side branch where it is unnecessary. | This invention provides a stent assembly with exterior banding for delivery of therapeutic agents that would overcome the previous disadvantages. Also this stent assembly which avoids side branch vessel blockage by a sheath, with increased drug storage capacity and allowing delivery of different drugs at different axial and radial locations. |
| 8 | US20040117007A1 | 06/17/2004 | STS Biopolymers, Inc. | Medicated stent having multi-layer polymer coating | The problem associated with previous stent and their implantation was thrombus formation in vicinity of stent. | The stent assembly developed in this invention consistently provide therapeutic activity from the surfaces of stents in order to reduce the incidence of restenosis and thrombus formation after coronary stenting procedures in the clinic. |
| 9 | WO1990013332A1 | 11/15/1990 | CEDARS-SINAI MEDICAL CENTER | STENT WITH SUSTAINED DRUG DELIVERY | Problem was to provide an intravascular stent that preserves vessel patency, inhibits luminal narrowing and at the same time stent can deliver a pharmaceutical agent to a specific body site or organ. | The stent assembly provided by this invention will release anticoagulants, antiplatelet drugs or drugs that inhibit excessive endothelial cell growth at the placement site, thereby preserving the vessels patency and inhibiting luminal narrowing. |
| 10 | WO2001036008A2 | 05/25/2001 | STS BIOPOLYMERS, INC. | MEDICAL DEVICES COATED WITH ELASTIC POLYMERIC MATERIAL | The lack of flexibility, expandability and lubricity of coating surface was the problem with previous insertable medical device. | This invention provides a medical device with a polymeric coating adherent to the covering, such that the coating and covering possess desirable surface characteristics such as lubricity, or lack thereof, as well as flexibility, expandability and elasticity. |
Dolcera Dashboard
Dashboard Link
| Ureteral Stent - Dashboard |
- Flash Player is essential to view the Dolcera Dashboard
- To access the Dashboard you have to signup. You can do so by clicking here
Patent Heat Map
Clinical Trials
New trials
| |
|
| ||
| |
Assessment of Drug-Eluting Ureteral Stent on Bacterial Adherence and Biofilm Formation | Renal Calculi, Ureteral Obstruction | Ureteral Stent | Lawson Health Research Institute, Boston Scientific Corporation |
| |
Memokath® 044TW Stent for Treatment of Urethral Stricture | Urethral Stricture | Memokath stenting | Engineers & Doctors Wallsten Medical Group |
| |
Study to Determine if There Are Specific Clinical Factors to Determine Stent Encrustation | Kidney Stones | N\A | University of California, Irvine |
| |
Ureteral Stent Length and Patient Symptoms | Kidney Stones | Ureteral Stent | Emory University |
| |
Drainage of Malignant Extrinsic Ureteral Obstruction Using the Memokath Ureteral Stent | Ureteral Obstruction | Memokath 051 Ureteral Stent | Mayo Clinic Engineers & Doctors Wallsten Medical Group |
| |
A Prospective Comparison Between Ureteral Stent and Nephrostomy Tube for an Urgent Drainage of Obstructed Kidney (JJVsPCN08) | Kidney Disease | Nephrostomy tube and ureteral stent | Rabin Medical Center |
Concluded trials
| Long-term outcome of permanent urethral stents in the treatment of detrusor-sphincter dyssynergia | To evaluate the long-term efficacy of a permanently implanted urethral stent in the treatment of spinally injured patients with detrusor-sphincter dyssynergia. | 13 | Detrusor-sphincter dyssynergia | Stenting is an effective alternative to sphincterotomy in the long-term, although secondary bladder neck obstruction is a frequent problem. | |
| |
Nephrostomy Tube or 'JJ' Ureteric Stent in Ureteric Obstruction: Assessment of Patient Perspectives Using Quality-of-Life Survey and Utility Analysis | Upper urinary tract obstruction is often relieved by either a percutaneous nephrostomy tube (PCN) or a ureteric stent. Both can cause considerable morbidity and reduce patient's health-related quality of life (QoL). We have compared the QoL in these 2 groups. | 34 | Upper urinary tract obstruction | Patients with 'JJ' stents have significantly more irritative urinary symptoms and a high chance of local discomfort than patients with nephrostomy tubes (PCN). However, based on the EuroQol analysis, there is no significant difference in the gross impact on the health-related QoL or the utility between these groups indicating no patient preference for either modality of treatment. |
| |
Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial. | A randomized, prospective trial to compare the incidence of early urological complications and health care expenditures in renal transplant recipients with or without ureteral stenting. | 201 | Renal transplant recipient | Using a ureteral stent at renal transplantation significantly decreases the early urinary complications of urine leakage and obstruction. However, there is a significant increase in urinary tract infections, primarily beyond 30 days after transplantation. Stent removal within 4 weeks of insertion appears advisable. |
Adverse Events
| Cook Urologicals Cook Urological Stent | Stent broke into pieces while removing it from the patients body. | 12/14/2005 | |
| Boston Scoientific Boston Scientific Ureteral stent System | Fractured stent seen under Fluroscopy | 10/17/2007 | |
| Boston Scoientific Boston Scientific Ureteral Stent System Kit 8 FR X 24 CM | During insertion of ureteral stent, the stent broke into multiple parts which were retained in the patient. | 10/14/2005 | |
| Boston Scientific Corp Boston Scientific 8 FR X 28 CM Ureteral Stent System Kit | Breakage of the upper loop of the ureteral stent while trying to insert it. | 01/05/2005 | |
| Boston Scientific Bostoon Scientific Micro Vasive Contour VL Ureteral Stent | Broken stent observed during x-ray procedure. | 12/12/2003 |
Pre-Market Notification
Section 510(k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical device at least 90 days in advance, also known as Premarket Notification. This premarket submission demonstrates to the FDA that the device to be marketed is atleast as safe and effective, that is, substantially equivalent, to a legally marketed device. Parties required to submit a 510(k) to the FDA include domestic or foreign manufacturers introducing a device to the U.S. market, as well as specification developers and repackers/relabelers.
A 510(k) is required when:
- Introducing a device into commercial distribution (marketing) for the first time.
- Proposed different intended use for a device already in commercial distribution.
- Change or modification of a legally marketed device.
510(k) “Substantial Equivalence” Decision Making Process
Some of the companies active in the field of ureteral stents have been represented in the table below.
| |
|
|
|
|
| ||
| |
|
|
|
|
Silicone | Double pigtail with monofilament suture loop | |
| |
|
|
|
Dual Durometer Percuflex with HydroPlus Coating | Bladder loop design | | |
| |
|
|
|
Metal | Temporary stenting | | |
| |
|
|
|
Polyurethane | Spiral radially expanding stent | | |
| |
| ||||||
| |
|
|
|
Nickel-titanium shape memory alloy | Double fluted ended spiral stent | |
Timeline Visualization
Products
The FDA classifies a ureteric stent as follows:
- TITLE 21 - FOOD AND DRUGS
- CHAPTER I - FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
- SUBCHAPTER H - MEDICAL DEVICES
- PART 876 - GASTROENTEROLOGY-UROLOGY DEVICES
- Subpart E - Surgical Devices
- Sec. 876.4620 - Ureteral stent.
- Classification - class II device Code of Federal Regulations
| Sr. No. | Company | Device(s) | Approval | Approval Date | Material | Technology | Indwelling Time (days) | Image |
| 1 | Allium, Israel | URS | CE Mark | Jul, 2007 | Nickel-titanium shape memory alloy covered by polymer | Self-expanding stent | File:Allium.png | |
| 2 | Pnn Medical A/S | Memokath 051 | CE Mark | 1995 | Nickel-titanium shape memory alloy | Double fluted ended spiral stent | 240 | |
| FDA Listing | Mar, 2004 | |||||||
| 3 | Fossa Medical | Stone Sweeper | CE Mark | Sep, 2005 | Polyurethane | Radially expanding stent | 13 | |
| FDA 510(k) | Aug, 2002 | |||||||
| Open lumen stent | FDA 510(k) | Nov, 2003 | Polyurethane | Pigtail-tipped stent with ’Pusher’ | ||||
| CE Mark | Sep, 2005 | |||||||
| Expanding Ureteral Stent | FDA 510(k) | Jun, 2002 | Polyurethane | Double pigtail stent with ’Pusher’ | N/A | |||
| 4 | Boston Scientific | Contour | Percuflex - proprietary polyolefin copolymer; Hydroplus coating | Fixed and variable length; Tapered tip | 365 | |||
| Percuflex | Percuflex | Pigtail | 365 | |||||
| Polaris Ultra | FDA 510(k) | Jan, 2001 | Dual Durometer Percuflex with HydroPlus Coating; soft Nautilus Bladder Coil. | Double pigtail | 365 | |||
| Polaris Loop | FDA 510(k) | Mar, 2003 | Dual Durometer Percuflex with HydroPlus Coating | Bladder loop design | 365 | |||
| Retromax Plus | Percuflex material and Hydroplus coating | Endopyelotomy stent | Post-procedure healing | |||||
| Stretch VL Flexima | Hydroplus Coating | Variable length coil on distal and proximal ends | 90 | |||||
| Drug-Eluting Stent | Percuflex - proprietary polyolefin copolymer | Ketorolac trimethamine loaded stent | N/A | |||||
| 5 | Cook Medical | Resonance | FDA 510(k) | May, 2007 | Metal | 365 | ||
| Sof-flex | AQ® Hydrophilic Coating | Radiopaque tip and tether for repositioning | 180 | |||||
| Endo-Sof | AQ® Hydrophilic Coating | Double pigtail | 365 | |||||
| C-Flex | Double Pigtail | 180 | ||||||
| Smith Universal | Nephrostomy tube + Ureteral stent | 60 | ||||||
| Endo-Sof Radiance | Launch | Dec, 2007 | Heparin-bonded stent | |||||
| 6 | Q Urological | pAguaMedicina™ Pediatric Ureteral Stent | FDA 510(k) | Jan, 2010 | Hydrogel | Differentially larger end (no pigtail) | 30 | |
| 7 | Bioteque Corp. | Ureteral Stent Set | FDA 510(k) | Apr, 2010 | 30 | |||
| 8 | Applied Medical Resources, CA, USA | Mesh | FDA 510(k) | Jul, 2001 | Polyester mesh | Double-pigtail | N/A | |
| Silhouette | Coil-reinforced; SL-6® hydrophilic coating | Patency Device | ||||||
| Applied Standard | FDA 510(k) | Jun, 1999 | Proprietary thermoplastic elastomer material; SL-6® hydrophilic coating | Unique wall construction and enlarged drainage holes | ||||
| 7-10 endopyelotomy | Proprietary thermoplastic elastomer material; SL-6® hydrophilic coating | Dual Diameter stent | ||||||
| 9 | Bard Urological | InLay Optima | FDA 510(k) | Dec, 2004 | Silicone | Double pigtail with monofilament suture loop | 365 | |
| Bardex® Double Pigtail Soft Stent | FDA 510(k) | Jan, 2003 | Silicone | Attached with suture for ease of removal | ||||
| Fluoro-4 Silicone Ureteral Stent | Silicone/tantalum | |||||||
| Figure-4 Silicone Ureteral Stent | Silicone | Three dimensional design | ||||||
| InLay Ureteral Stent | FDA 510(k) | Dec, 1998 | Silicone | Tapered tip and lubricious hydrophilic coating | ||||
| Urinary Diversion Stent | FDA 510(k) | Apr, 1991 | Silicone | |||||
| 10 | Coloplast-Porges | Vortek | FDA 510(k) | Oct, 1998 | Silicone | Double coating for easy maneuverability as well as flexibility | ||
| Biosoft | FDA 510(k) | Oct, 1998 | Silicone | Extreme flexibility | ||||
| Polyurethane | Hard or soft Polyurethane | Designed for short-term use | 90 | |||||
| Silicone | FDA 510k) | Oct, 2002 | Silicone | Pyatiprofilnaya technology | ||||
| 11 | Teleflex Medical | Rüsch Superglide DD | FDA 510(k) | Jul, 1999 | WIRUTHAN® (polyurethane) with hydrogel coating | Directable and detachable | ||
| 12 | Gyrus ACMI/Cabot/Acromed/Circon/Surgitek | Classic closed-tip | FDA 510(k) | Dec, 1986 | Classic Closed Tip | |||
| Classic Double pigtail | FDA 510(k) | Mar, 1996 | Tecoflex® construction | Balanced-curled double pigtail design | ||||
| Double-J | FDA 510(k) | Apr, 1988 | Silicone | Double-J closed-tip | ||||
| Lithostent | Tecoflex® | Grooved design | ||||||
| Lubri-flex | FDA 510(k) | Nov, 1991 | Tecoflex® | “Rememberance” of shape with a chemically bonded wettable solution | ||||
| Multi-flex | Tecoflex® | Two durometers with helical kidney curls | ||||||
| Quadra-Coil multi-length | FDA 510(k) | Mar, 1996 | Tecoflex® | Accomodate ureteral lengths from 22cm to 28cm | ||||
| Sof-curl | Tecoflex® | Dual-durometer design and exclusive soft bladder helix | ||||||
| Uroguide | Silicone | Classic Double J with open tip | ||||||
| 13 | Ameco Medical Industries | Amecath | Nitinol; Available with hydrophilic coating | Double loop stent | Short-term and long-term | |||
| 14 | Angiomed-Movaco (C.R. Bard subsidiary) | Ureteral Stent Set | FDA 510(k) | Jan, 1987 | Nitinol | Self-expanding stent | N/A |
Patent-Product-Clinical Trial Mapping
- To access the Dashboard you have to signup. You can do so by clicking here
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
Product to Patent Mapping
- Click here to download the excel file.
- Mapped Patent vs Not Mapped Patents
Key Findings
Major Players
- Boston Scientific Limited, Abbott, Medtronic and Cook Inc. are the major players in ureteral stent research field.
Key Patents
- The key patents in the field are held by Cook Incorporated, Interventional Thermodynamics, Abbott laboratories and Boston Scientific Corp.
IP Activity
- Patenting activity has been high growth rate during the period 2001 to 2005 with a peak no. of patents in year 2005, followed by saturation during the period 2006 to 2008 and after that a gradual declination upto year 2010 in the ureteral stent research area.
Geographical Activity
- USA, Europe and Germany are very active in ureteral stent research.
- References
1- http://en.wikipedia.org/wiki/Demographics_of_the_United_States
2- http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink
3- http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf
Trends in stent R&D
- Given that the first bare metal stent entered the market in 1994, the technology is far from mature.
- Whilst drug eluting stents will be improved through the use of what are described as more ‘cell friendly’ (i.e. more hydrophobic) coatings and adapted (by using CFD in stent design) to varying wall sheer conditions, the industry is already well down the road of developing bioresorbable coatings and even bioresorbable stents.
- Such systems are already on limited trial in Europe with FDA trials aimed at 2012 and approval by 2015.
- There are still other areas under study including thinner struts which are important in terms of giving improved stent flexibility, a reduced cross-sectional profile within the vessel and potentially reduced restenosis (due to reduced vascular trauma).
- Silicon carbides, carbon coatings, titanium nitride/oxide and sputtered indium oxide have all been trialled as routes to reducing the incidence of restenosis and/or thrombosis.
- Click here for recent news and developments in Boston Scientific and Cook INC
Source Ceram-newsrelease
Competitor Analysis
Total Sales in 2010 - 4.04 Billion USD
| Company | Total Sales in 2010 | Urological sales | Percentage share | Product portfolio |
| Boston Scientific | 7800 | 661 | 8.48 | Boston_portfolio |
| CR BARD | 2700 | 702 | 26.00 | BARD_portfolio |
| Cook Medical | 1700 | - | - | Cook_portfolio |
| Medline | 4040 | - | - | Medline_portfolio |
All figures in USD million
Boston Scientific
Net sales in 2010 – USD 7.8 Billion
Share of Urology- 8.48 %
Net sales from Urology- USD 661 Million
Source BSsalesdata
CR BARD
Net sales in 2010 – USD 2.7 Billion
Share of Urology- 26%
Net sales from Urology- USD 702 Million
Source – CR BARD annual report
Cook Medical
Total Sales - USD 1.7 Billion
Medline
Academic Research
Research at University of Minnesota about symptoms and pain associated with ureteral stent
Background and Purpose
- Ureteral stents are associated with significant pain and urinary symptoms.
- Manufacturers have altered stent designs and materials in an attempt to minimize this morbidity.
- This study evaluated the impact of these modifications.
Patients and Methods
- Stent manufacturers were asked to provide the 6F ureteral stent they believed would be associated with the least patient discomfort.
- Patients undergoing uncomplicated ureteroscopy were randomized to the Bard Inlay, Cook Endo-Sof, Microvasive Contour, Applied Medical Vertex, or Surgitek Classic Double-Pigtail stent.
- The Ureteric Stent Symptom Questionnaire (USSQ) was administered on days 1, 3, and 5, and the patients maintained a narcotic diary. The data were analyzed using ANOVA and nonparametric methods.
Results
- A total of 44 patients (73%) completed all USSQ questionnaires.
- Urinary symptom scores were significantly lower for the Inlay stent on day 3 than for the Vertex (P = 0.01), Contour (P = 0.05), Endo-Sof (P = 0.03), and Classic (P = 0.02) stents. No significant differences were noted in pain and general symptom scores or narcotic use.
Conclusion
- The Bard Inlay stent is associated with less-severe urinary symptoms than other ureteral stents.
- The USSQ is a sensitive tool to measure differences between stents.
The complete USSQ form is available at BUI and endourology.
Source University of Minnesota
Removed Sections
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Addition
Patent Categorization: Interactive mind map linked to Dolcera Dashboard
- To access the Dashboard you have to signup. You can do so by clicking here
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
Product-Patent-Clinical Trials Mapping
- To access the Dashboard you have to signup. You can do so by clicking here
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
Product to Patent Mapping
- Click here to download the excel file.
- Mapped Patent vs Not Mapped Patents
Dolcera Dashboard
Dashboard Link
| Ureteral Stent - Dashboard |
- Flash Player is essential to view the Dolcera Dashboard
- To access the Dashboard you have to signup. You can do so by clicking here