Difference between revisions of "Osteoporosis"
From DolceraWiki
Manikandan (Talk | contribs) (→Products in market) |
Manikandan (Talk | contribs) (→Contact Dolcera) |
||
| Line 851: | Line 851: | ||
* [http://www.asbmr.org/meeting/index.cfm American Society of Bone and Mineral Research Annual Meeting, September 12-16, 2008, Montreal, Canada] | * [http://www.asbmr.org/meeting/index.cfm American Society of Bone and Mineral Research Annual Meeting, September 12-16, 2008, Montreal, Canada] | ||
| + | ==<span style="color:#C41E3A">Like this report?</span>== | ||
| + | <p align="center"> '''This is only a sample report with brief analysis''' <br> | ||
| + | '''Dolcera can provide a comprehensive report customized to your needs'''</p> | ||
| + | {|border="2" cellspacing="0" cellpadding="4" align="center" " | ||
| + | |style="background:lightgrey" align = "center" colspan = "3"|'''[mailto:info@dolcera.com <span style="color:#0047AB">Buy the customized report from Dolcera</span>]''' | ||
| + | |- | ||
| + | | align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services Patent Analytics Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/business-research-services Market Research Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools/patent-dashboard Purchase Patent Dashboard] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Landscape Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/research-processes Dolcera Processes] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/industries Industry Focus] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Search Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/alerts-and-updates Patent Alerting Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools Dolcera Tools] | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
==Contact Dolcera== | ==Contact Dolcera== | ||
Latest revision as of 22:49, 5 August 2009
Osteoporosis is a systematic skeletal disease characterized by low bone mass, increase of bone fragility and susceptibility to fracture.
Types of Osteoporosis and its causes
- Primary osteoporosis(Type I (postmenopausal) or Type II (senile) ):
- Type I (postmenopausal): Generally develops in women after menopause when the amount of estrogen in the body greatly decreases. This process leads to an increase in the resorption of bone (the bones loses substance).
- Type II (senile): This involves a thinning of both the trabecular bone (the spongy bone inside of the hard cortical bone) and the hard cortical bone. This process often leads to hip and vertebral body (in the spine) fractures.
- Secondary osteoporosis: Secondary osteoporosis has the same symptoms as primary osteoporosis. However, it occurs as a result of having certain medical conditions, such as hyperthyroidism or leukemia. It may also occur as a result of taking medications known to cause bone breakdown, such as oral or high-dose inhaled corticosteroids (if used for more than 6 months), too high a dose of thyroid replacement, or aromatase inhibitors (used to treat breast cancer).
- Osteogenesis imperfecta: Osteogenesis imperfecta is a rare form of osteoporosis that is present at birth. Osteogenesis imperfecta causes bones to break for no apparent reason.
- Idiopathic juvenile osteoporosis:Idiopathic juvenile osteoporosis is rare. It occurs in children between the ages of 8 and 14 or during times of rapid growth. There is no known cause for this type of osteoporosis, in which there is too little bone formation or excessive bone loss.
Contents
Incidence and prevalence
Incidence
- 1 in 3 women over 50 will suffer a fracture due to osteoporosis; this increases to 1 in 2 over 60.
- 1 in 5 men over 50 will suffer a fracture due to osteoporosis; this increases to 1 in 3 over 60.
- Approximately 1.6 million hip fractures occur each year worldwide, the incidence is set to increase to 6.3 million by 2050.
- The highest risk of hip fractures are seen in Norway, Sweden, Iceland, Denmark and the USA.
- Currently, there is an increasing incidence of hip fractures in the developed cities in Asia. 1 out of 4 hip fractures occur in Asia and Latin America. This number of hip fractures will increase to 1 in 2 by 2050.
- In the Middle East, the burden of osteoporosis in the general population is expected to increase and is becoming a heavy financial burden.7
- The annual incidence rate of osteoporotic fractures in women is greater than the combined incidence rates of heart attack, stroke and breast cancer. (International osteoporosis foundation)
Prevalence
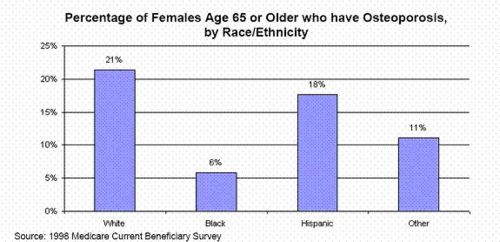
- 28 million Americans (10 million with osteoporosis; 18 million with low bone mass); eight million American women and 2 million men (NWHIC)(approx 1 in 9 or 10.29% or 28 million people in USA ).
- Total prevalence rate of osteoporosis in the middle- aged and elderly in China was 16. 1% in 2002. The prevalence rate among males was 11. 5% and among females was 19. 9%.
- Prevalence increase to 2.2 million in 2006 and 3 million in 2021 in Australia.
- The prevalence of Khon Kaen (Thai rural area) women has osteporosis in femoral neck and lumbar spine is 19.3% and 24.7% respectively. (International osteoporosis foundation)
| S.NO | Country/Region | Extrapolated Prevalence |
| 1 | USA | 10 million cases (80% cases are women and 20% cases are men) |
| 2 | Australia | 2.2 million |
| 3 | Middle east | 18,603,593 |
| 4 | Africa | 47,233,590 |
| 5 | Asia | 359,862,754 |
| 6 | Europe | 80,590,570 |
Product Information
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Products in market
| Generic | Brand | Current Status | Type | Company |
| Antiresorptives | ||||
| Bisphosphonates | ||||
| Alendronate | FOSAMAX® | Approved by US | Postmenopausal | Merck |
| Risedronate | Actonel® | Approved by US/ES | Postmenopausal | Sanofi-Aventis/Procter & Gamble |
| Ibandronate | Boniva™ | Approved by US | Postmenopausal | Roche |
| Etidronate | Didronel | Off Label | Proctor And Gamble | |
| Zoledronic Acid | Aclasta | Approved | Postmenopausal | Novartis |
| Selective Estrogen Receptor Modulators | ||||
| Raloxifene | Evista® | Approved by US | Osteoporosis | Eli lilly |
| Hormone Replacement Therapy | ||||
| Conjugated estrogen | Premarin® | Approved by US | Postmenopausal | Wyeth |
| Conjugated estrogen | Cenestin™ | Approved by US | Postmenopausal | Duramed |
| Esterified estrogens | Estratab® | Approved by US | Postmenopausal | Solvay |
| Esterified estrogens | Menesta | Approved by US | Postmenopausal | Monarch |
| Estradiol Transdermal System | Menostar | Approved by US | Postmenopausal | Bayer |
| Estradiol Transdermal System | Vivelle Dot | Approved by US | Postmenopausal | Noven |
| Estradiol | Estrace® | Approved by US | Postmenopausal | Bristol-Myers Squibb |
| Estradiol | Gynodiol | Approved by US | Postmenopausal | Novavax |
| Tibolone | Livifem | Postmenopausal | ||
| Calcitonin | ||||
| Calcitonin Salmon Injection | Miacalcin® | Approved by US | Postmenopausal | Novartis |
| Calcitonin Salmon Nasal Spray | Miacalcin® | Approved by US | Postmenopausal | Novartis |
| Calcitonin Salmon Nasal Spray | Fortical® | Approved by US | Postmenopausal | |
| Bone Forming Drugs | ||||
| Teripratide | Forteo® | Approved by US | Osteoporosis | Pfizer |
| Drugs with Complex Mechanisms of Action | ||||
| Strontium Ranelate | Protelos | Not approved in USA | Postmenopausal osteoporosis | Servier |
| Vitamin D | ||||
| Calcium | FDA is considering amending rules so that companies can now claim that calcium and Vitamin D in their food supplements can prevent osteoprorosis in all ages and both sexes | FDA | ||
Product pipeline
| Product Pipeline | ||||
| DRUG | COMPANY | DESCRIPTION | STAGE | |
| RANKL Inhibitors | ||||
| Denosumab | Atlantos, Amgen | A monoclonal antibody | 3 | |
| Bisphosphonates | ||||
| Minodronate (YM529) | Astellas | Nitrogen containing bisphosphonate | ||
| Calcium Antagonist | ||||
| 423557 | Glaxosmithkline | 1 | ||
| 423562 | Glaxosmithkline | 1 | ||
| Calcilytics (751689) | NPS and Glaxosmithkline | Calcilytics antagonize calcium receptors on parathyroid glands resulting in a transient release of the body’s own stores of parathyroid hormone (PTH). | 1 | |
| Parathyroid Hormone | ||||
| 768974 | Glaxosmithkline | Parathyroid Hormone Agonist | 1 | |
| Preos (rDNA origin) | NPS | Recombinant Parathyroid Hormone | Registration Filed | |
| PTH1-34 | Nastech | Intranasal Parathyroid hormone | 2 | |
| PTH1-34 | Novartis, Emisphere | Oral Parathyroid hormone | 1 | |
| PTH Analogs | Unigene | 2 | ||
| Cathepsin k Inhibitor | ||||
| Relacatib (SB-462795) | GlaxoSmithkline | 1 | ||
| AAE581 | Novartis | |||
| MK-0822 | Canadian Institutes of Health Research, Merck | 2 | ||
| Selective Estrogen Receptor Modulators | ||||
| Bazedoxifene | Wyeth, Ligand | A Third-Generation Selective Estrogen Receptor Modulator for Treatment of Postmenopausal Osteoporosis | ||
| Lasofoxifene (Oporia) | Pfizer,Ligand | A Third-Generation Selective Estrogen Receptor Modulator for Treatment of Postmenopausal Osteoporosis | NDA | |
| Arzoxifene | Eli lilly | |||
| Taromifene (Fareston) | Orion | |||
| Calcitonin | ||||
| Nastech | NDA | |||
| Salmon calcitonin | Unigene | Nasal | 3 | |
| Salmon calcitonin | Unigene | Oral | 2 | |
| Salmon calcitonin (SMC 021) | Novartis, Emisphere, Nordic | Oral | 3 | |
| Salmon calcitonin | Nobex (Biocon) | Oral | 3 | |
| Vitamin D | ||||
| ED 71 | Bone/ Chugai | Activted Vitamin D derivative | 3 | |
| Doxercalciferol (Hectorol) | Bone Care Int. | Synthetic Vitamin D | ||
| Nitrates: Research is going on to determine if nitrates can prevent osteoporosis in women. | ||||
| Isosorbide mononitrate | St. Michael’s Hospital, Toronto | 3 | ||
| Nitroglycerine | Canadian Institutes of Health Research (CIHR) | 3 | ||
| HCT 1026 (NO releasing Derivative of NSAIDs) | NicOx SA | 2 | ||
| Selective Androgen Receptor Modulator (SARM) | ||||
| LGD-2941 | Ligand, TAP | 3 | ||
| LGD-3303 | Ligand, TAP | 3 | ||
| Ostarine | GTx | 2 | ||
| Intermittent Versus Continuous Androgen Suppression | Ontario Cancer Research Network | |||
| Mk 0733 | Merck | 1 | ||
| TH0229 | Theratechnologies | |||
| c-8500 | Merck | 2 | ||
| c-3578 | Merck | 2 | ||
| apomorphine | Ilex Oncology | 2 | ||
| Genistein | Primus Pharmaceuticals, Inc. | isoflavones have been shown to interact with animal and human estrogen receptors, causing effects in the body similar to those caused by the hormone estrogen. | Available | |
| Soy estrogens | (NIAMS) | 3 | ||
| Hesperidin | Nestle clinical nutrition | 3 | ||
| Davallia Divaricata | National Taiwan University Hospital | 1 | ||
| Atorvaststin | [1] | |||
| Potassium citrate | Weill Medical College of Cornell University | 4 | ||
| Phosphorus | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) | 3 | ||
Devices
| Hip Protector | Hip pads with special underwear with pockets | Children’s Mercy Hospital Kansas City |
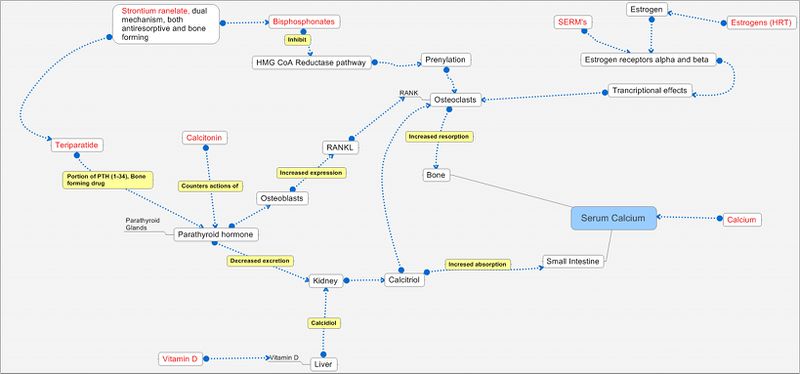
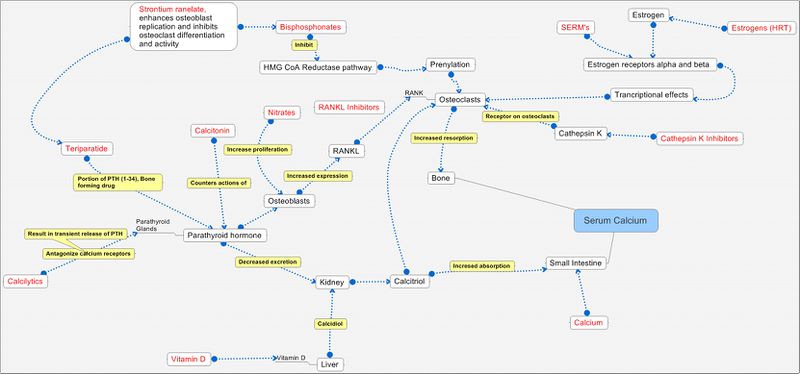
Pathways
Market Information
| Prescription Trends in USA | |||||||||
| Source: Arch Intern Med. 2004;164:1525-1530 | |||||||||
| 1988 | 1990 | 1992 | 1994 | 1996 | 1998 | 2000 | 2002 | 2003 | |
| Patients (Millions) | NA | 0.6 | 0.6 | 0.5 | 1.2 | 2 | 2.6 | 2.8 | 3.6 |
| Drugs Prescribed % | |||||||||
| Bisphosphonates (total) | 1 | 9 | 17 | 14 | 48 | 49 | 54 | 71 | 73 |
| Alendronate | 0 | 0 | 0 | 0 | 45 | 47 | 48 | 53 | 51 |
| Risedronate | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 17 | 22 |
| Etidronate | 1 | 9 | 17 | 14 | 3 | 1 | 1 | 0 | 0 |
| Calcium | 39 | 33 | 33 | 43 | 31 | 26 | 29 | 26 | 24 |
| Calcitonins | 5 | 9 | 10 | 13 | 13 | 16 | 15 | 7 | 5 |
| Estrogens | 35 | 25 | 33 | 27 | 17 | 12 | 8 | 5 | 3 |
| SERMs | 0 | 0 | 0 | 0 | 0 | 9 | 14 | 12 | 12 |
Clinical Trials
| Trial No. | Condition | Description | Intervention | Therapy | Route of admn | Sponsors & Collaborators | Phase | Date |
| 1 | Postmenopausal Osteoporosis | Investigate Patient Preference On Dosing In Ibandronate And Risedronate in korean women | Ibandronate | Drug | Oral | GlaxoSmithKline | Phase IV | May-07 |
| 2 | Osteoporosis Osteopenia |
Texture Analysis for Postmenopausal Osteoporosis | Alendronate | Drug | Oral | University of Chicago | - | Mar-07 |
| 3 | Osteoporosis | A Phase II Study Evaluating SB-751689 in Post-Menopausal Women With Osteoporosis. | SB-751689 | Drug | Oral | GlaxoSmithKline | Phase II | May-07 |
| 4 | Osteoporosis | Fall prevention program | Procedure | - | - | Sint Maartenskliniek ZonMw: The Netherlands Organisation for Health Research and Development |
Apr-07 | |
| 5 | Osteoporosis Osteopenia |
High Dosage Vitamin D and Osteoporosis | Cholecalciferol | Drug | Oral | University Hospital of North Norway | Phase IV | Jun-07 |
| 6 | Osteoporosis | Effect of PTH(1-84)or Strontium Ranelate on Bone Formation Measured by Bone Markers | Parathyronine hormone | Drug | Oral | Nycomed | Phase IV | May-07 |
| 7 | Post-Menopausal Osteoporosis | A Study of Quarterly Intravenous Bonviva (Ibandronate) in Women With Post-Menopausal Osteoporosis. | ibandronate [Bonviva/Boniva] | Drug | Intravenous | Hoffmann-La Roche | Phase IV | Jul-07 |
| 8 | Osteoporosis | A Study for Patients With Osteoporosis | 1.Salmon Calcitonin 2.Teriparatide | Drug | Nose/intravenous | Eli Lilly and Company | Phase III |
Jun-07 |
| 9 | Osteoporosis | Safety/Efficacy of Zoledronic Acid and Alendronate on Bone Metabolism in Post Menopausal Women With Osteoporosis | Zoledronic acid and alendronate | Drug | Oral | Novartis | Phase III |
May-07 |
| 10 | Osteoporosis | Efficacy in Reducing Fractures and Safety of Zoledronic Acid in Men With Osteoporosis | zoledronic acid | Drug | Intravenous | Novartis | Phase III |
Feb-07 |
| 11 | Osteoporosis | Davallia Divaricata BL: The Use of Traditional Chinese Native Medicine for Osteoporosis | Gu-Sui-Bu (Davallia Divaricata) | Drug | National Taiwan University Hospital | Phase I |
Jan-07 | |
| 12 | Osteoporosis | Dose Ranging Study - Macroflux PTH in Postmenopausal Women With Osteoporosis | Macroflux PTH | Drug | Subcutaneuos | The Macroflux Corporation | Phase II | Jun-07 |
| 13 | Osteoporosis | Fosamax for Childhood Cancer Survivors | Alendronate | Drug | Oral | Chinese University of Hong Kong | Phase III | Oct-06 |
| 14 | Osteoporosis | This study is currently recruiting patients. | Parathyroid Hormone (PTH) | Drug | Subcutaneuos | Nycomed | - | Feb-07 |
| 15 | Osteoporosis | This study is currently recruiting patients. | Alendronate with cholecalciferol and Os-Cal with extra D | Drug | Oral | National Institute on Aging (NIA),Glaxosmithkline | Phase III | May-07 |
| 16 | Primary Osteoporosis | Phase II/III Clinical Study of R484iv (Ibandronic Acid) for Primary Osteoporosis | Ibandronic acid | Drug | Oral | Chugai Pharmaceutical | Phase III | Mar-07 |
| 17 | Postmenopausal Osteoporosis, Back pain and Spinal Fracture |
Effect of Teriparatide Compared to Risedronate on Back Pain in Women With a Spine Fracture Caused by Osteoporosis | Teriparatide and risedronate | Drug | Oral/Subcutaneuos | Eli Lilly and Company | Phase III | Jul-07 |
| 18 | Osteoporosis | BONDIR Study: A Study of Intravenous Bonviva (Ibandronate) After Recent Vertebral Osteoporotic Fracture in Patients With Osteoporosis. | Ibandronate [Bonviva/Boniva] | Drug | Intravenous | Hoffmann-La Roche | Phase III | Jul-07 |
Intellectual Property
Search strategy
- Database/Vendor: Micropat
- Search scope: US Granted US Applications EP-A EP-B WO JP (bibliographic data only) DE-C,B DE-A DE-T DE-U GB-A FR-A; Claims
- Years: Issue/Publication Date: >20051231
- Search String: ((Osteoporosis OR (bone Near3 regeneration)) AND (treatment))
- Hits: 1261/982 patents (with/with out family members)
- Date of search: 14th September 2007
Top players and IPC classes
Geographical Distribution and Treatment Approaches
IP activity and Treatment Approaches
- Decline in the IP activity during 2006 and 2007 is due 18 months publication delay of patent document.
Conferences
- International Conference on Osteoporosis and Bone Research to be held on Oct 15-18, 2008 in Beijing, China.
- 2008 IOF World Congress on Osteoporosis to be held on December 2-7, 2008, Bangkok, Thailand
- 2007 World Congress on Osteoarthritis, December 6-9, 2007, Marriott Harbor Beach Resort & Spa in fabulous Ft. Lauderdale, Florida
- American Society of Bone and Mineral Research Annual Meeting, September 12-16, 2008, Montreal, Canada
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Contact Dolcera
| Samir Raiyani |
|---|
| Email: info@dolcera.com |
| Phone: +1-650-269-7952 |