Difference between revisions of "Inflammation and cardiovascular drugs"
Abhay.goel.1 (Talk | contribs) (→Targeting inflammation – Pharmaceutical focus) |
Abhay.goel.1 (Talk | contribs) (→Targeting inflammation – Pharmaceutical focus) |
||
| Line 5: | Line 5: | ||
Inflammation is one of the key processes linked to various diseases and disorders. There are many opportunities for designing and developing specific new drugs for inflammatory responses. | Inflammation is one of the key processes linked to various diseases and disorders. There are many opportunities for designing and developing specific new drugs for inflammatory responses. | ||
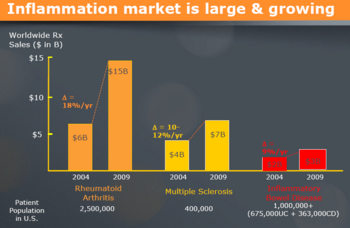
| − | [[image:market.png|center| | + | [[image:market.png|center|1200 px|thumb|[http://www.chromos.com/documents/roadshowsept14_003.pdf Source]]] |
---- | ---- | ||
Revision as of 08:16, 20 April 2012
Contents
[hide]- 1 Targeting inflammation – Pharmaceutical focus
- [+]2 Overview of inflammation
- [+]3 Cardiovascular diseases
- [+]4 Drug targets
- [+]5 Drug targets for cardiovascular diseases
- [+]6 Like this report?
- [+]7 Intellectual property - G-proteins and cardiovascular diseases
- [+]8 Research activities
- 9 Like this report?
- 10 Contact Dolcera
Targeting inflammation – Pharmaceutical focus
The pharmaceutical industry is one of the most profitable sectors of the Fortune 500 with profits of approximately 18% of revenue compared to a median of 5% for other industry segments. However, this market is facing increasing competitive pressure due to escalating R&D costs, shortened patent life due to the long product approval process, increased sales and marketing costs, pricing pressures, and entrance of more competitors into the industry. It is estimated that large pharmaceutical companies (or Pharma as they are generally referred to in the industry) require 3-5 new lead drug candidates (defined as New Chemical Entities or NCEs) each year to sustain doubledigit growth rates. Despite the fact that R&D costs have been increasing (typically $300-500 million per NCE), the average number of NCEs have been dropping over the last decade to under one per year across the industry. Thus, there is increasing pressure on Pharma to find newer discovery and development paradigms.
Inflammation is one of the key processes linked to various diseases and disorders. There are many opportunities for designing and developing specific new drugs for inflammatory responses.
Overview of inflammation
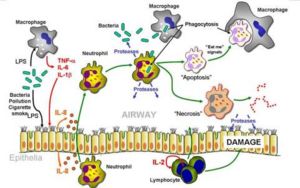
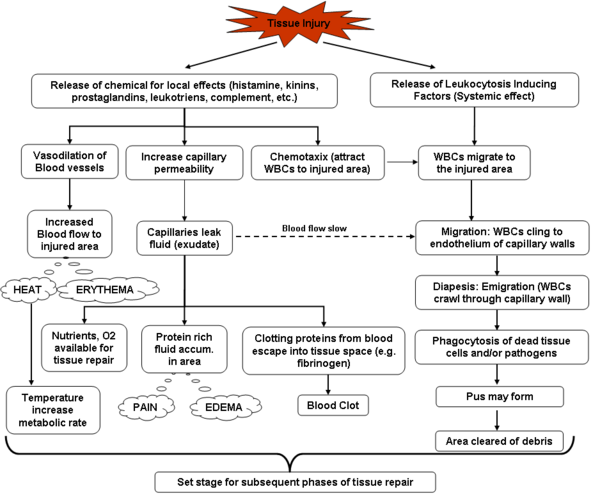
Inflammation is a process by which the body’s white blood cells and chemicals protect us from infection and foreign substances such as bacteria and viruses.In some diseases, however, the body’s defense system (immune system) inappropriately triggers an inflammatory response when there are no foreign substances to fight off, these are called autoimmune diseases, where the body’s normally protective immune system causes damage to its own tissues. When inflammation occurs normally, chemicals from the body’s white blood cells are released to protect us from foreign substances.
Inflammation is characterized by:
- increased blood flow to the tissue causing
- increased temperature,
- redness,
- swelling, and
- pain.
Chronic inflammatory diseases afflict millions of people across the world leading to untold suffering, economic loss and premature death. Thease includes rheumatoid arthritis, osteoarthritis and inflammatory lung disease, including inflammatory bowel disease, atherosclerosis and psoriasis.
Chronic inflammatory disorders are a substantial burden in social and economic terms for the Australian community. Arthritis alone afflicts 3.4 million Australians and the financial costs were in excess of $19.25 billion in 2004 (Access Economics 2005).
Despite the importance of these diseases, there have been relatively few innovative breakthroughs into their cause, treatment or cure, despite intensive global research.
Various inflammatory diseases
- Rheumatoid arthritis (RA) is a disease that most commonly affects the joints, but can sometimes also cause damage to other organs. RA is an auto-immune disease, that is, a disease where a person’s immune system attacks his or her own body tissues. This auto-immune reaction causes inflammation of the joints, particularly of the synovial membrane which lines them. There is an over-production of synovial (joint) fluid and this, combined with the inflammation, causes joints to become swollen and painful.
- Chronic Obstructive Pulmonary Disease, or COPD, is a group of progressive lung diseases characterized by airflow obstruction or limitation that is not fully reversible. The restricted airflow is generally progressive and associated with abnormal inflammatory response of the lungs to irritants." They are most commonly caused by tobacco smoking. The family of diseases includes chronic bronchitis, emphysema and bronchiectasis. COPD develops slowly and it may be many years before symptoms such as breathing difficulties are noticed. Thus COPD is usually diagnosed in middle-aged or older people. COPD is also characterized by exacerbations which are often associated with a rapid progression of symptoms such as shortness of breath, wheezing, and sputum production, sometimes leading to respiratory failure. Exacerbations are most commonly brought on by infectious agents. Bronchodilators, antibiotics, and oral or intravenous steroids are used to treat these episodes.
- Osteoarthritis (OA) is the most common form of arthritis and is characterised by mild to debilitating pain, which can involve almost any joint but, in particular, weight bearing joints such as the hip, knee, spine and feet. OA refers to a degeneration of the articular cartilage that makes up the joint surface. This breakdown removes the soft buffer between the bones and can, when severe, result in bone against bone friction, which can cause severe pain and loss of movement.
- Inflammatory Bowel Disease (IBD) is the name of a group of disorders that cause the intestines to become inflamed (red and swollen). IBD can be painful and debilitating and causes chronic inflammation of the digestive tract. The two most common forms of IBD are ulcerative colitis and Crohn’s Disease. Both conditions inflame the lining of your digestive tract and both can cause severe bouts of watery diarrhoea and abdominal pain.
- Psoriasis is a common immune-mediated chronic skin disease that comes in different forms and differing levels of severity. It is a condition that is generally found on the knees, elbows, scalp, hands, feet or lower back, and generally appears as patches of raised red skin covered by a flaky white build up. It can cause intense itching and burning.
- Artherosclerosis is the term for the process of fatty substances, cholesterol, cellular waste products, calcium and fibrin building up in the inner lining of an artery. It is a slow and progressive disease that may start in childhood. If left untreated, artherosclerosis can lead to heart attack or stroke. The first symptom of a narrowing artery may be pain or cramps at times when the blood flow can’t keep up with the body’s demand for oxygen. For example, during exercise a person may feel chest pain because of a lack of oxygen to the heart or while walking, a person may feel leg cramps because of a lack of oxygen to the legs.
- Pelvic inflammatory disease (PID) is a general term that refers to infection and inflammation of the upper genital tract in women. It can affect the uterus (womb), fallopian tubes (tubes that carry eggs from the ovaries to the uterus), ovaries, and other organs related to reproduction. The scarring that results on these organs can lead to infertility, tubal (ectopic) pregnancy, chronic pelvic pain, abscesses (sores containing pus), and other serious problems.
- Cardiovascular diseases: C-reactive protein (CRP) is one of the acute phase proteins that increase during systemic inflammation. Recent studies also suggest that higher levels of hs-CRP may increase the risk that an artery will reclose after it’s been opened by balloon angioplasty. Most studies show that the higher the hs-CRP levels, the higher the risk of developing heart attack. Source
- Metabolic disease: Scientists at Galileo Pharmaceuticals, Inc. have demonstrated a direct relationship between inflammation and glucose levels. Inhibiting lipoxygenases, known mediators of inflammatory response, significantly lowers blood glucose levels in animal models of diabetes. Lipoxygenases are enzymes which play a key role in the synthesis of inflammatory mediators called leukotrienes, and trigger the progression of several diseases mediated by inflammation. Two key members of the lipoxygenase pathway, 5-lipoxygenase (5-LO) and 15-lipoxygenase (15-LO) have been indicated in a number of diseases states including asthma, arthritis, atherosclerosis, diabetes, osteoporosis, and cancer. Source
- Neurodegenerative disorder: Alzheimer's disease, the most common neurodegenerative disorder, is characterized by aggregates of fibrillar Aß derived from misprocessing of amyloid precursor protein (APP). Aß deposits are surrounded by activated microglia and astrocytes, which have been postulated to contribute to Alzheimer's pathophysiology by establishing a chronic inflammatory state. Source
Factors associated with inflammations
| Players of inflammation | Description |
| Mast Cells | Mast cells appear to be key players in the initiation of inflammation. Mast cells are found in the tissues. Their cytoplasm is loaded with granules containing mediators of inflammation. Their surface is coated with a variety of receptors which, when engaged by the appropriate ligand, trigger exocytosis of the granules. Source |
| Tumor Necrosis Factor-alpha (TNF-α) | Large amounts of TNF-α are quickly released by stimulated mast cells. All the cells involved in inflammation have receptors for TNF-α, and are activated by it to synthesize more on their own. This positive feedback quickly amplifies the response. Source |
| Chemokines | These are chemotactic cytokines; that is, secreted proteins that attract other leukocytes into the area. Source |
| Reactive Oxygen Species (ROS) | These are produced by activated phagocytes: macrophages and neutrophils. They are toxic for microorganisms but can also lead to tissue injury. Source |
| Histamine | The granules of mast cells are loaded with histamine and their exocytosis releases this potent mediator. Histamine increases the blood flow to the area and the leakage of fluid and proteins from the blood into the tissue space. Thus the quick release of histamine is largely responsible for the redness and swelling associated with inflammation. Source |
| Interleukin-1 (IL-1) | Macrophages and monocytes are the main source of this cytokine. IL-1 has both • paracrine effects on cells in the vicinity, e.g., • causing them to produce tissue factor and thus triggering the blood clotting cascade. • stimulating the synthesis and secretion of a variety of other interleukins • helping to activate T cells and thus initiate an adaptive immune response • endocrine (hormonal) effects as it is carried in the blood throughout the body. • decreasing blood pressure • inducing fever. IL-1 causes fever by stimulating the release of prostaglandins, which act on the temperature control center of the hypothalamus. Source |
| Inflammasomes | IL-1 is synthesized from a larger precursor that is cleaved by a caspase (caspase-1). Caspase-1 is part of two (or more) multi-protein complexes in the cytosol of macrophages and neutrophils that are called inflammasomes. Inflammasomes are activated by several different products produced by invading bacteria. Some of these are first "seen" by toll-like receptors (TLRs) thus providing a link between the innate immune system and inflammation. Source |
| Leukotrienes and Prostaglandins | These potent mediators of inflammation are derivatives of arachidonic acid (AA) a 20-carbon unsaturated fatty acid produced from membrane phospholipids. The principal pathways of arachidonic acid metabolism are • the 5-lipoxygenase pathway, which produces a collection of leukotrienes (LT) and • the cyclooxygenase (COX) pathway, which produces prostaglandin H2 (PGH2). PGH2 serves as the substrate for two enzymatic pathways: one leading to the production of several • prostaglandins (PG); the other leading to the production of • thromboxanes (Tx). Source |
Current treatment approaches
| Treatment approach | Description |
| Steroids | Like the glucocorticoid cortisol. Source |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | The NSAIDs achieve their effects by blocking the activity of cyclooxygenase. NSAIDs also inhibit clotting. They do this by interfering with the synthesis of thromboxane A2 in platelets. Source |
| COX-1 and COX-2 | The body produces several different forms of cyclooxygenase (COX), including • COX-1, which is involved in pain, clotting, and protecting the stomach; • COX-2, which is involved in the pain produced by inflammation. COX-2 inhibitors are effective against inflammation and seem to avoid damage to the GI tract. But, unfortunately, they increase the risk of blood clots — which can cause heart attacks and strokes — because they do not block the synthesis of thromboxane A2 by platelets (which contain only COX-1). Source |
| Therapeutic Proteins | Recombinant DNA and monoclonal antibody technology have produced some new therapies that are being enlisted in the battle against damaging inflammation. • an IL-1 antagonist that binds and inactivates the IL-1 receptor. • etanercept (Embrel®). A soluble version of the TNF-α receptor. It binds TNF-α preventing it from carrying out its many inflammatory actions. Potent but carries a severe risk of allowing infections to develop. • recombinant protein C. To help the body dissolve the tiny clots that are triggered during inflammation. • infliximab (Remicade®). A monoclonal antibody that binds to TNF-α. Shows promise against some inflammatory diseases such as rheumatoid arthritis. (Side-effect: can convert a latent case of tuberculosis into active disease.) Source |
Key signaling pathways
| Pathways | Activation | Key molecules | Target disease | Description |
| MAPK Pathways | Stress and Inflammation | Chronic inflammation, heart disease, stroke, diabetes mellitus | Source | |
| JNK Pathways | Cytokines or environmental stress | Cellular proliferation, apoptosis, and tissue morphogenesis | Source | |
| Cytokine Pathways | TNF-alpha, IL-1, IL-6, IL-10, IL-4 | Joint Inflammation in Rheumatoid Arthritis | Source | |
| NF-κB pathway | Diverse signal transduction cascades | IκB kinases, IKKα and IKKβ | Inflammation and cancer | Source |
| Protein Kinase Cascades |
Mitogens but by cellular stresses and inflammatory cytokines | ERKs, MEKs, SAPKs, Ras/MAPK, p38 | Stress and Inflammation | Source |
| TNF Pathways | TNF-R1 | Sepsis, diabetes, cancer, osteoporosis, multiple sclerosis, rheumatoid arthritis, and inflammatory bowel diseases | Source | |
| p38 Pathway (MAPK pathway) | Bacterial pathogens and cytokines | (ERKs, JNK/SAPK, ERK5/big MAP kinase 1 (BMK1), p38 group of protein kinases | Inflammation, cell growth, cell differentiation, the cell cycle, and cell death | Source |
| TRAF6-NFκB | TLR/IL-1R, CD40, transcription factors NFκB and AP1 | Autoimmune disease | Source | |
| STAT4 Pathways | Chemokines | Allergic Airway Inflammation | Source | |
| Toll-like receptors (TLRs) Pathways | Cytokine-driven inflammation and tissue destruction | Toll-like receptors (TLRs) | Rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, chronic obstructive pulmonary disease, and atherosclerosis | Source |
| Multiple signaling pathways | C-reactive protein, ICAM-1, VCAM-1), fibronectin, selectins, interleukin-1, heparin sulfate, Clotting or coagulation factors, Fibrinolysis factors, angiotensin II, Prostaglandins, TGF-[beta], (PDGF, NO and endothelin-1 | Atherosclerosis and cardiovascular disease | Source | |
| G protein-coupled receptors (GPCRs) Pathways | RGS proteins, PI3K | Cardiovascular diseases | Source |
Cardiovascular diseases
- It has become clear that CVD is an inflammatory disease, although what triggers the inflammation is still unknown.
- Diagnosis of CVD through inflammatory markers is an active area of research. Although several classes of anti-hypertensive drugs exist, there are still several treatment goals that remain to be achieved, such as better blood pressure control, greater protection against organ damage, and better tolerability.
- Several angiogenic and myogenic stem cell therapies are under investigation and promise in the long run to advance cardiac rehabilitation and even prevent heart failure. Despite the development of improved therapies, millions of Americans continue to live with life-threatening cardiovascular diseases.
Market analysis
- The total worldwide cardiovascular market is expected to show revenues of $91.2 billion in 2008, an increase of 6.9% compared with 2003.
- CVD is the leading cause of death in most regions throughout the world. Cardiovascular drugs constitute the world's largest pharmaceutical sector, with annual global sales over $70 billion.
- Risk factor management for CVD is an emerging market. Developing more effective ways of delivering pharmacological, nutritional, and preventative therapies are recognized as important targets that are under continual development. Aging populations and dietary and lifestyle induced disease processes ensure a growing need for cardiovascular devices.
- Reducing the costs for such devices by appropriately treating cardiovascular risk factors has become a major focus of the pharmaceutical and healthcare industries. Here are just some of the considerable market opportunities within the CVD Market:
- Increased use of cholesterol-controlling statins will cause prices to fall and generic brands to appear as knowledge of their benefits spread among general practitioners.
- Develop anti-glycosylation therapies to prevent the cross-linking that weakens aging heart muscle, and to reduce the diabetic risk factor.
- Create new vaccines to raise high density lipoprotein (HDL) levels, which help stave off heart disease.
- Develop new angiotensin receptor blockers to offer greater competition against ACE inhibitors
- Develop angiogenesis drugs such as VEGF (vascular endothelial growth factor) that will obviate the need for much bypass surgery by growing new arteries on damaged hearts.
Common cardiac drugs
| Common cardiac drugs | |||
| Drugs | Main effects | Mechanism | Sites of action |
| Abciximab | Anticoagulant stops platelet activation | Monoclonal antibody to fibrinogen receptors | Platelets |
| Amiloride (combination with frusemide is frumil) | Potassium sparing diuretic | Plasmalemma sodium & chloride channels | Kidney (distal tubules) |
| Amiodarone | Class III anti-arrhythmic | Prolongs action potential duration | Myocardium |
| Aspirin | Anticoagulant stops platelet activation | COX inhibitor, blocks TXA2 synthesis | Platelets |
| Atropine (sometimes used to stop vagus bradycardia) | Parasympatholytic, increases heart rate | Blocks muscarinic acch receptors | Pacemaker cells (sino-atrial node) |
| Captopril | Reduces arterial blood pressure | ACE inhibitor | Relaxes vascular smooth muscle |
| Clopidogrel | Anticoagulant stops platelet activation | Blocks ADP receptor | Platelets |
| Digitalis and ouabain | Increase cardiac contractility, delay AV node triggering | Block Na / K atpase raising intracellular sodium, then calcium | All tissues, but the Na/Ca exchanger is mainly in heart |
| Dipyridamole (often used for X-ray imaging) | Coronary vasodilation | Inhibition of adenosine uptake | Coronary vasculature |
| Furosemide (= frusemide) | Diuretic | Plasmalemma sodium & chloride channels | Kidney (loop of Henle) |
| Isoprenaline (and other adrenaline analogues) | Increase cardiac contractility | Beta agonist raises cyclic AMP | Many tissues |
| Losartan | Reduces arterial blood pressure | Angiotensin AT1 receptor blockade | Relaxes vascular smooth muscle |
| Lovastatin | Reduces blood cholesterol levels | HMG-coa reductase inhibitor | Liver |
| Morphine | Pain relief (mainly) | Opiate receptors | Brain |
| Nitroglycerine (and many other organic nitrates) | Reduce cardiac work load | Metabolised to NO | Relaxes vascular smooth muscle |
| Propranolol | Reduces cardiac contractility, class II anti-arrhythmic | Beta blocker lowers cyclic AMP | Many tissues |
| Quinidine, novocaine and other local anaesthetics | Class I anti-arrhythmics | Delay recovery of sarcolemma sodium channels after AP | Myocardium |
| Spironolactone (usually added to other diuretics) | Reduces diuretic potassium losses | Aldosterone antagonist | Kidney (distal tubules) |
| Urokinase (streptokinase is cheaper but antigenic) | Dissolves blood clots (fibrinolytic) | Activates plasminogen to plasmin (protease) | Blood clots |
| Verapamil, nifedipine and other dihydropyridines | Reduce cardiac work load, class IV anti-arrhythmic | Block sarcolemma calcium channels | Myocardium; relax vascular smooth muscle |
| Warfarin | Anticoagulant Vit. K antagonist |
Blocks -carboxy glutamate synthesis | Liver |
Drug targets
Target molecule (or target protein) are the molecule on which pharmaceutical researchers focus when designing a drug. Often, the target molecule is from a virus or bacterium, or is an abnormal human protein. In these cases, the researchers usually seek to design a small molecule— a drug— to bind to the target molecule and block its action.
Emerging drug targets
Drug targets are generally proteins. Nucleic acids are possible drug targets, and approaches such as RNA interference (RNAi) may indeed have advantages over proteins, but they have not so far been developed extensively. Protein targets consist mainly of enzymes, regulatory molecules such as cytokines, receptors for hormones or other regulatory molecules, transporters, and ion channels. Proteins that interact with small molecules are considered the most promising targets, because it is often possible to design or discover small molecule activators or inhibitors based on the natural ligands. Such substances are relatively straightforward to develop as drugs. Overall, enzyme targets account for over 50% of marketed drugs, and GPCR targets represent more than 20%, including 23 of the 100 best-selling drugs in the US. Hence the other target classes between them make up the remaining 25-30% of marketed drugs. Aberrant signal transduction plays a key part in the pathology of many serious diseases including cancer, inflammatory, cardiovascular, metabolic, and neuropsychiatric diseases. Proteins involved in signal transduction pathways therefore represent important drug targets. Three prominent superfamilies of proteins involved in signal transduction are: GPCRs, protein kinases, and nuclear receptors. Source
Classification of drug targets

The introduction of genomics, proteomics and metabolomics has paved the way for biology-driven process, leading to plethora of drug targets. The list of potential drug targets encoded in a genome includes most natural choice of virulent genes and species-specific genes. Other options include targeting RNA, enzymes of the intermediary metabolism, systems for DNA replication, translation apparatus or repair and membrane proteins.
- Species-specific genes as drug targets
- RNA as drug target
- Nucleic acid as drug targets
- Proteins as drug targets
- Membranes as drug targets
The comparison between the categories and numbers of drug targets in the years 2001 and 2005 shows the influence of genomics on drug discovery-the addition of new drug targets. Source
| Drug targets | 2001 | 2005 |
| GPCRs | 30% | 20% |
| Ion Channel | 20% | 30% |
| Enzymes | 30% | 20% |
| Hormones/Vitamins | 12% | 4% |
| RNA-Proteins/DNA-Proteins | - | 10% |
| Protein-Protein | - | 20% |
| Unknown Mechanism | 8% | - |
Drug targets for cardiovascular diseases
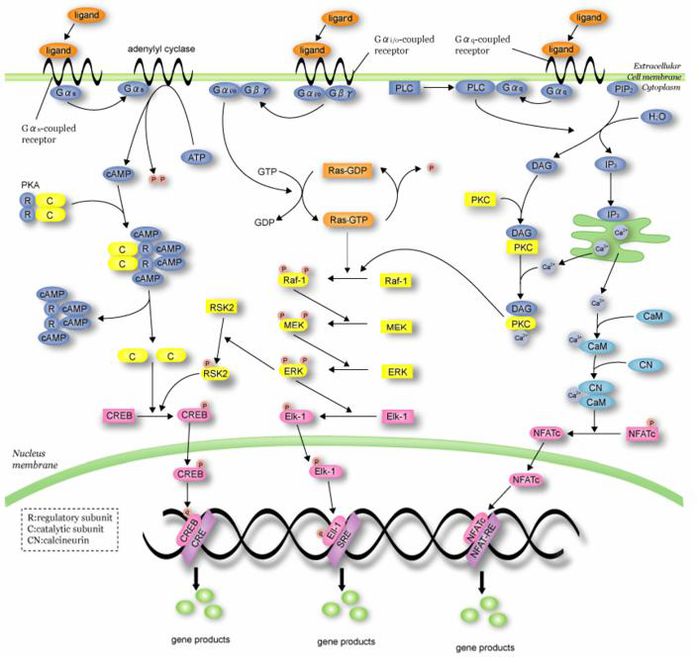
G- protein-coupled receptors (GPCRs)
GPCRs are the largest and most important class of receptors in humans because they are the targets of more than half of all drugs used clinically, especially for treating diseases of the cardiovascular and nervous systems. GPCRs are also the targets of many drugs of abuse.
G Protein-Coupled Receptors (GPCRs) account for 50% of the current drug targets, and remain about one-third of the drug discovery effort in the drug discovery-development community. As a result of the sequencing of the human genome, more than 150 orphan and non-chemosensory GPCRs have been identified for which their cognate ligands or biological functions are unknown. De-orphanization of those receptors could bring novel therapeutic targets to the industry against which novel therapeutics could be designed. In addition, current GPCR-based drugs only target ~30% of the approximately 200 known GPCRs in the genome. This is a rich target class that is highly-druggable. Source
Today, more than 50% of drug targets are based on GPCRs and the annual worldwide sales exceeds 50 billion dollars. GPCRs are involved in all major disease areas such as:
- Cardiovascular,
- Metabolic,
- Neurodegenerative,
- Psychiatric,
- Cancer and
- Infectious diseases.
The major efforts established through large networks of structural genomics on GPCRs, where recombinantly expressed GPCRs are subjected to purification and crystallization attempts with the intention of obtaining high-resolution structures, are a promising future approach for tailor-made drug development. Source
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
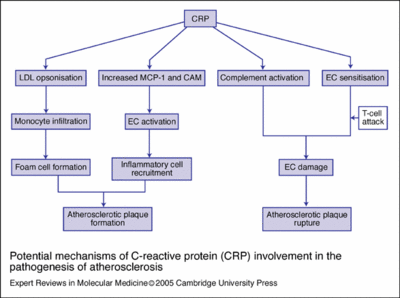
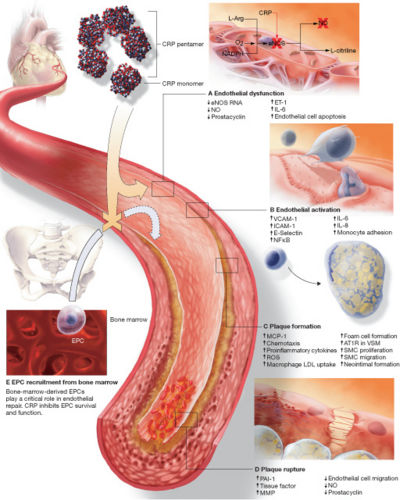
C-reactive protein (CRP)
C-reactive protein (CRP) is a plasma protein, an acute phase protein produced by the liver. It is a member of the pentraxin family of proteins. Recent research suggests that patients with elevated basal levels of CRP are at an increased risk for diabetes[4], hypertension and cardiovascular disease. [1]
CRP a host-defensive role, as phosphocholine is found in microbial polysaccharides (where CRP-binding activates the classical complement pathway and opsonises ligands for phagocytosis), the pro-inflammatory platelet-activating factor (PAF) (which is neutralised), and polymorphs (which are down-regulated). Source
The role of C-reactive protein in atherogenesis
(A) Endothelial dysfunction
(B) Endothelial cell activation
(C) Plaque formation
(D) Plaque rupture
(E) Inhibition of EPC survival and function
Treatment approaches - Lowering level of CRP
- Vitamin E
- Vitamin B6
- Aspirin
- Gugulipid
- Proteolytic Enzymes (VRP’s UniZyme formula)
- Other anti-inflammatory compunds (Herbs, Turmeric and Boswellia, and fish oil) Source
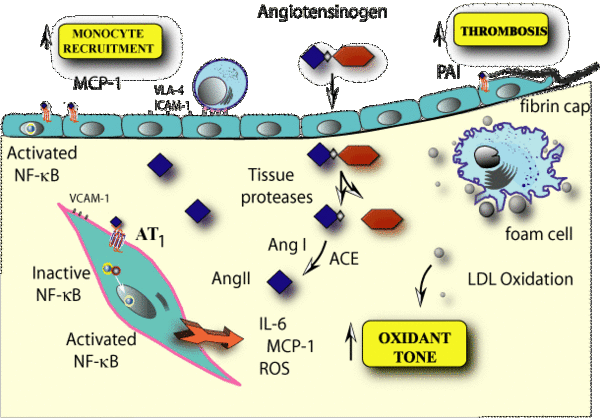
Renin-angiotensin system
Vascular inflammation is now recognized to be an independent risk factor for the development of atherosclerosis. Chronic elevations of circulating IL-6 or its biomarkers are predictors for increased risk for the development or progression of ischemic heart disease. Our laboratory has been investigating the role of the renin-angiotensin system in accelerating vascular inflammation. The potent vasopressor peptide, angiotensin II, induces the expression of cardioactive cytokines, such as IL-6, in vascular smooth muscle cells. We are particularly interested in the role of the NF-kB transcription factor in signaling the inflammatory response in the vessel wall. In this project, we are pursuing the hypothesis that NF-kB is a central mediator of inflammation and atherosclerosis in the large vessels. Source
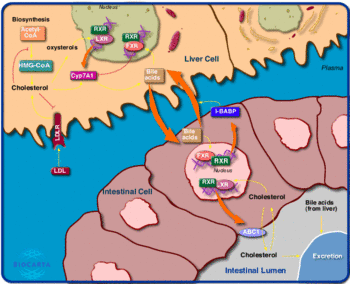
LXR signaling pathways
The liver X receptors {alpha} and {beta} (LXR{alpha} and LXR{beta}) are members of the nuclear receptor family of proteins that are critical for the control of lipid homeostasis in vertebrates. The endogenous activators of these receptors are oxysterols and intermediates in the cholesterol biosynthetic pathway. LXRs serve as cholesterol sensors that regulate the expression of multiple genes involved in the efflux, transport and excretion of cholesterol. Recent studies have outlined the importance of LXR signaling pathways in the development of metabolic disorders such as hyperlipidemia and atherosclerosis. Synthetic LXR agonists inhibit the development of atherosclerosis in murine models, an effect that is likely to result from the modulation of both metabolic and inflammatory gene expression. These observations identify the LXR pathway as a potential target for therapeutic intervention in human cardiovascular disease. Source
Cholesterol is essential for life and a key in the development of heart disease. Cholesterol homeostasis is achieved through regulation of cholesterol uptake, cholesterol biosynthesis, cholesterol conversion to bile acids and excretion of bile acids. Inhibition of cholesterol biosynthesis upregulates LDLR expression and is the mechanism of action of many drugs used to lower plasma LDL to reduce coronary heart disease. Many aspects of cholesterol homeostasis are regulated by the nuclear receptors FXR and LXR, both nuclear receptor transcription factors that form heterodimers with the retinoic acid RXR receptors and that are activated by cholesterol metabolites. One of the primary tissues in cholesterol metabolism is the liver, a key site of cholesterol biosynthesis and where cholesterol low-density lipoprotein (LDL) is taken up from the plasma by the LDL-receptor. When cholesterol accumulates in liver cells, some of the cholesterol is oxidized to create oxysterols. Oxysterols activate LXR through LXR/RXR heterodimers to activate genes such as the CYP7A1 enzyme that catalyzes the rate-limiting step in bile acid biosynthesis and a major route for the elimination of cholesterol. Animals lacking the CYP7A1 enzyme accumulate cholesterol in the liver. In the intestine LXR activates the ABC-1 gene, a transporter that actively transports cholesterol out of cells to clear it from the body. Activation of ABC-1 expression by LXR in macrophages in atherosclerotic plaques appears to be another mechanism by which LXR plays a role in heart disease. The FXR receptor is activated by bile acids. In the liver, activation of FXR-RXR heterodimers by bile acids results in the feedback inhibition of CYP7A expression and reduced biosynthesis of bile acids. In the intestine, FXR activates expression of I-BABP, a protein that increases the transport of bile acids back to the liver from the intestine, reducing their excretion. Drugs targeting the FXR and LXR receptors could play an important role in modulating cholesterol homeostasis and heart disease in the future. Source
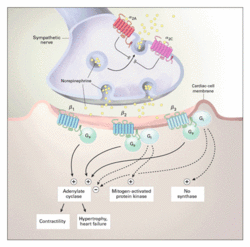
Cardiac Adrenergic Signaling
The adrenergic receptors are G-protein–coupled transmembrane receptors. On binding to ligands, the G proteins dissociate from the intracellular domain of the receptor to propagate signals by modulating the activity of downstream effector molecules such as adenylate cyclase, phospholipases, and ion channels. The {beta}1-Adrenergic receptor and {beta}2-adrenergic receptor are both normally coupled to the stimulatory G protein (Gs), and through this protein, they activate adenylate cyclase, augmenting myocyte contractility and inducing myocyte hypertrophy or heart failure. This regulation of cell growth involves multiple downstream molecules including mitogen-activated protein kinases in a complex regulatory network. {beta}2-Adrenergic receptors may also couple to the inhibitory G protein (Gi) and reduce adenylate cyclase activity while activating the mitogen-activated protein kinase pathway. The role of the {beta}3-adrenergic receptors is less clear, but they seem to activate nitric oxide synthase through Gi. {alpha}-Adrenergic signaling also has an important role in cardiac physiology. When stimulated, {alpha}1-adrenergic receptors (not shown) are potent mediators of cardiomyocyte hypertrophy, acting through another subtype of G protein (Gq), which stimulates mitogen-activated protein kinase pathways. The two subtypes of {alpha}2-adrenergic receptors ({alpha}2A and {alpha}2C) depicted here operate as presynaptic inhibitors of norepinephrine release, but are also known to have postsynaptic functions. The {alpha}2A-adrenergic receptor is important for central and peripheral nervous system inhibition of norepinephrine release at high action-potential frequencies, whereas the {alpha}2C-adrenergic receptor is more important for regulating lower-frequency stimulation. These {alpha}2-adrenergic receptors inhibit the release not only of norepinephrine but also of other neurotransmitters that are not ligands for the adrenergic receptors. Source
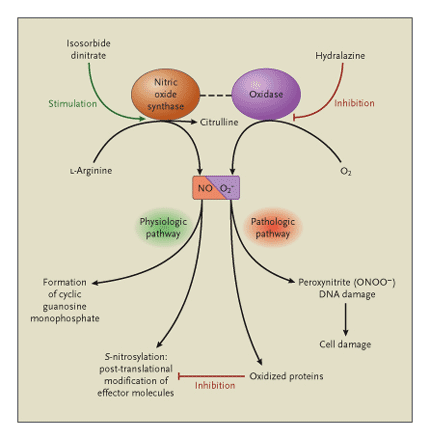
Nitroso–Redox Balance
The interaction between nitric oxide (NO) and superoxide (O2–) production — the nitroso–redox balance — plays fundamental roles in cell and organ failure at key sites throughout the cardiovascular system. Physiologically, the level and location of nitric oxide and superoxide production are balanced within the cell, facilitating appropriate post-translational modification of effector proteins. In patients with heart failure, the production of superoxide is increased and the level or location of nitric oxide synthesis is disrupted, interrupting effector signaling and thus causing cellular dysfunction as a result of vasoconstriction of small and large blood vessels and cardiac contractility or, if prolonged, cell death or damage. Isosorbide dinitrate, a drug that stimulates the nitric oxide pathway, and hydralazine, an antioxidant that inhibits superoxide synthesis, may therefore restore the balance of nitric oxide and superoxide production, converting the pathologic pathways to physiologic pathways in both the heart and the blood vessels. Source
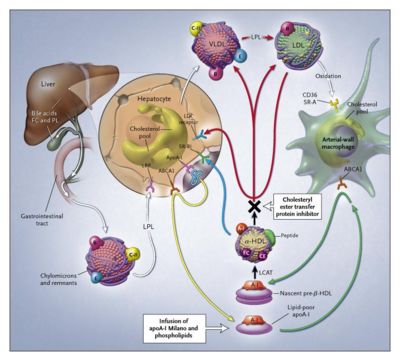
HDL Cholesterol Levels
Triglycerides and cholesterol are transported by chylomicrons and remnant lipoproteins from the intestine and by very-low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) from the liver (white arrows). Apolipoprotein A-I (apoA-I) is synthesized by the liver and, after interaction with hepatic ATP-binding cassette transporter 1 (ABCA1), is secreted into plasma as lipid-poor apolipoprotein A-I (yellow arrow). In reverse cholesterol transport, newly synthesized lipid-poor apolipoprotein A-I interacts with ABCA1, removing excess cellular cholesterol and forming pre-{beta}-HDL (green arrow). Pre-{beta}-HDL is converted into mature {alpha}-HDL by lecithin–cholesterol acyltransferase (LCAT, black arrow). HDL cholesterol is returned to the liver through two pathways: selective uptake of cholesterol by the hepatic scavenger receptor, class B, type I (SR-BI, blue arrow), or the transfer of cholesteryl ester by cholesteryl ester transfer protein (CETP) to VLDL–LDL, with uptake by the liver through the LDL receptor (red arrows). Short-term HDL therapy to increase the HDL level and potentially provide protection against cardiovascular events can be achieved with the infusion of complexes consisting of apolipoprotein A-I Milano and phospholipids. Long-term increases in the HDL level and reductions in the LDL level result from the partial inhibition of CETP. FC denotes free cholesterol, PL phospholipids, LRP LDL-related protein, and LPL lipoprotein lipase. Source
Intellectual property - G-proteins and cardiovascular diseases
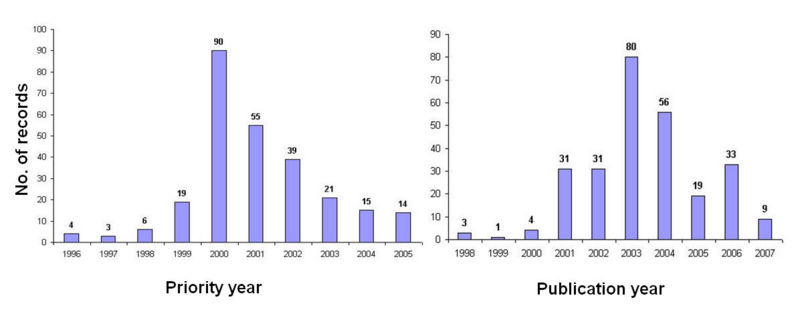
IP activity
IP activity (based on priority year) in the G- protein-coupled receptors (GPCRs) space initiated during the year 1996, and is continuously increasing, with highest activity during the year 2000.
NOTE: Decline in activity in the graph is due to the 18 months period for the patent publication.
Competitor's activity
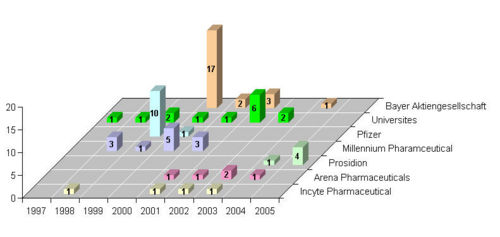
Bayer Aktiengesellschaft, Pfizer, Millennium Pharmaceutical are the top players.
Technology classification based on IPC class
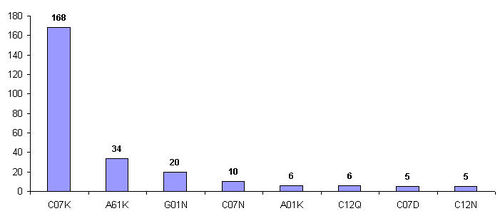
Most of the patenting activity is edivent in IPC class "C07K", pertains to peptides.
| Top IPC class | Definition |
| C07K | Peptides |
| A61K | Preparation for medical purposes |
| G01N | Investigating or analyzing materials by determining their chemical or physical properties |
Interactive signaling pathways and patents
Interactive signaling pathway of G- protein represented below with information on few patents targeting different sites. Click on the Hypertext molecules in the main pathway, to visualize the patent details.
Research activities
Recent research progress (year 2007) in the area of G-proteins is illustrated below, with interactive gene linking.
Future potential areas
- In vivo activation of 'G{alpha}q/PKC pathways could upregulate GRK2 expression in cardiovascular cells, thus contributing to the increased desensitization of ß-adrenergic and other GPCR systems, is of potential physiological relevance. Detailed mechanisms of regulation of GRK2 expression in different types of cells of the cardiovascular system, particularly in cardiac cell lines, and the determination of possible changes in GRK2 levels in VSMCs of patients with cardiovascular diseases are interesting fields for future research and may help to develop new therapeutic strategies based on the modulation of GRK2 expression. [2]]
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Contact Dolcera
| Samir Raiyani |
|---|
| Email: info@dolcera.com |
| Phone: +1-650-269-7952 |