Difference between revisions of "Template - Production Of Therapeutic Glycoproteins"
| Line 7: | Line 7: | ||
=Table of Contents= | =Table of Contents= | ||
| − | 1. Introduction | + | 1. Introduction<br> |
| − | 1.1. Glycosylation | + | 1.1. Glycosylation<br> |
| − | 1.2. Importance of Glycosylation in Therapeutic Proteins | + | 1.2. Importance of Glycosylation in Therapeutic Proteins<br> |
| − | 1.3. Glycosylation and Biopharmaceutical Manufacturing | + | 1.3. Glycosylation and Biopharmaceutical Manufacturing<br> |
| − | 1.4. Glycosylation in Biosimilars | + | 1.4. Glycosylation in Biosimilars<br> |
| − | 2. Search Strategy | + | 2. Search Strategy<br> |
| − | 3. Sample patent analysis | + | 3. Sample patent analysis<br> |
| − | 4. Taxonomy | + | 4. Taxonomy<br> |
| − | 5. Top Citations | + | 5. Top Citations<br> |
| − | 5.1. Patent Ranking | + | 5.1. Patent Ranking<br> |
| − | 5.2. Top Cited Patents | + | 5.2. Top Cited Patents<br> |
| − | 6. Patent to product mapping | + | 6. Patent to product mapping<br> |
| − | 7. Key Findings | + | 7. Key Findings<br> |
| − | 7.1. Major Player | + | 7.1. Major Player<br> |
| − | 7.2. IP Activity | + | 7.2. IP Activity<br> |
| − | 7.2.1. IP Activity based on Publication year | + | 7.2.1. IP Activity based on Publication year<br> |
| − | 7.2.2. IP Activity based on Priority year | + | 7.2.2. IP Activity based on Priority year<br> |
| − | 7.3. Geographical Activity | + | 7.3. Geographical Activity<br> |
| − | 7.3.1. Geographical Activity of Patents & their Family members | + | 7.3.1. Geographical Activity of Patents & their Family members<br> |
| − | 7.4. Categorization on the basis of production methods | + | 7.4. Categorization on the basis of production methods<br> |
| − | 7.4.1. Mammalian cell lines | + | 7.4.1. Mammalian cell lines<br> |
| − | 7.4.2. Transgenic Animals | + | 7.4.2. Transgenic Animals<br> |
| − | 7.4.3. Plants | + | 7.4.3. Plants<br> |
| − | 7.4.4. Microorganisms | + | 7.4.4. Microorganisms<br> |
| − | 7.4.5. Enzymes | + | 7.4.5. Enzymes<br> |
| − | 7.4.6. Chemical/Cell free synthesis | + | 7.4.6. Chemical/Cell free synthesis<br> |
| − | 7.4.7. Fermentation/culture conditions | + | 7.4.7. Fermentation/culture conditions<br> |
| − | 8. Conclusion | + | 8. Conclusion<br> |
| − | 9. References | + | 9. References<br> |
| − | 10. Appendix A: Control patents | + | 10. Appendix A: Control patents<br> |
| − | 11. Appendix B: Relevant class code definitions | + | 11. Appendix B: Relevant class code definitions<br> |
| − | 12. Appendix C: Concept table | + | 12. Appendix C: Concept table<br> |
13. Appendix D: Search strategy | 13. Appendix D: Search strategy | ||
| − | |||
=Methodology= | =Methodology= | ||
Latest revision as of 05:32, 21 November 2011
Contents
Objective
- The objective is to create a technology landscape report in the area of "Production of Therapeutic Glycoproteins"
Comment
The idea is to find out the innovative market players in this technology area, those are the players that are the most technologically advanced in the market place. Intellectual Property that these players hold is taken as a measure of their technological prowess in this particular area. We have used Patents to point out the innovative and technologically advanced market players.
Table of Contents
1. Introduction
1.1. Glycosylation
1.2. Importance of Glycosylation in Therapeutic Proteins
1.3. Glycosylation and Biopharmaceutical Manufacturing
1.4. Glycosylation in Biosimilars
2. Search Strategy
3. Sample patent analysis
4. Taxonomy
5. Top Citations
5.1. Patent Ranking
5.2. Top Cited Patents
6. Patent to product mapping
7. Key Findings
7.1. Major Player
7.2. IP Activity
7.2.1. IP Activity based on Publication year
7.2.2. IP Activity based on Priority year
7.3. Geographical Activity
7.3.1. Geographical Activity of Patents & their Family members
7.4. Categorization on the basis of production methods
7.4.1. Mammalian cell lines
7.4.2. Transgenic Animals
7.4.3. Plants
7.4.4. Microorganisms
7.4.5. Enzymes
7.4.6. Chemical/Cell free synthesis
7.4.7. Fermentation/culture conditions
8. Conclusion
9. References
10. Appendix A: Control patents
11. Appendix B: Relevant class code definitions
12. Appendix C: Concept table
13. Appendix D: Search strategy
Methodology
| Search Strategy | 1. Various keywords are retrieved for conducting the search related to production of therapeutic glycoproteins from pubmed mesh, relevant patents, scientific articles and thesaurus. 2. The database used for patent search is Thomson innovation. |
| Keywords | Glycosylation, therapeutic proteins, monoclonal antibody, hormone, production, cell culture, fermentation etc. |
Introduction
Glycosylation:
Post-translational modifications – and in particular glycosylation – play a decisive role in ensuring proper protein function. Glycosylation has been found to occur in a large variety of protein classes, including – but not limited to – antibodies, protein hormones, growth factors, cytokines and vaccines. Glycosylation is the covalent attachment of a carbohydrate component to the polypeptide backbone. For some proteins glycosylation can increase solubility, influence biological half-life and/or biological activity. The two main classes of glycosidic bonds to polypeptide chains are termed N-linked and O-linked.
- N-linked glycosylation: the glycan is linked to nitrogen in the side chain of asparagine. The oligosaccharide is attached via the amide nitrogen of an asparagine residue in the consensus sequence "asparagine — any amino acid except proline — serine/ threonine"
- O-linked glycosylation: the glycan is linked to oxygen in the side chain of mostly serine or threonine, as well as hydroxylysine. No specific consensus sequence has been identified for O-linked glycosylation.
In the case of humans, the oligosaccharides are typically composed of the following monosaccharides: mannose (Man), glucose (Glc), N-acetylglucosamine (GlcNAc), fucose (Fuc), galactose (Gal), N acetylgalactosamine (GalNAc), and sialic or neuraminic acids. The monosaccharides are linked together in a linear or branching fashion via either α- or β-glycosidic bonds between specific carbon atoms of the individual monosaccharides.Walsh G et al, Nature Biotechnology,2006
Importance of Glycosylation in Therapeutic Proteins:
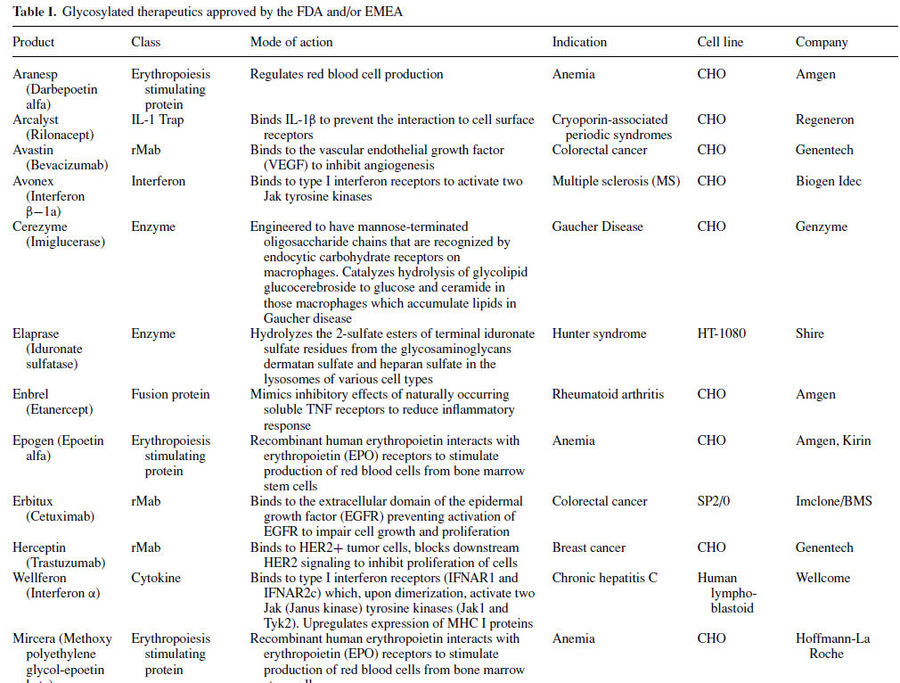
Many important therapeutic proteins are glycosylated including epoetin (EPO), granulocyte macrophage colony stimulating factor (GM-CSF) and tissue plasminogen activator (t-PA). Following table lists glycosylated therapeutics currently on the market.
Glycosylation is important because it can influence the biological activity of a protein through different mechanisms. First of all, glycosylation can affect half-life by influencing the active clearance of a protein. Certain pituitary glycoprotein hormones such as lutropin are rapidly removed from circulation by the liver if they bear a sulphated GalNAc residue. A second example is EPO, the serum half-life of which is dependent on four sialylated N-glycans (sialic acid is itself a glycan). Poorly glycosylated forms of EPO are rapidly cleared by filtration in the kidney. In addition, under-sialylated EPO is rapidly removed by hepatocytes and macrophages. Thus, increasing the degree of sialylation and glycosylation decreases the renal clearance rate, and can increase EPO in vivo activity.
Glycosylation can affect the activity of a recombinant protein more directly. Rituximab, for instance,is a monoclonal antibody (mAb) used to treat non-Hodgkin’s lymphoma through targeting of B-cell CD20 antigens. Its activity can be assessed with the antibody-dependent cellular cytotoxicity (ADCC) test, which measures the ability of an engineered mAb to lyse target cells. The ADCC of two types of mAb (one of which was rituximab) was found to vary inversely according to the mAb’s glycosylation state. Rituximab was produced from Chinese hamster ovary (CHO) cells with a relatively high level of glycosylation and was found to be several-fold less cytotoxic in vitro than another anti-CD20 mAb (KM3065), which was synthesized with fewer glycan residues from a rat cell line.Kullmann M et al,Nephrol Dial Transplant. 2006
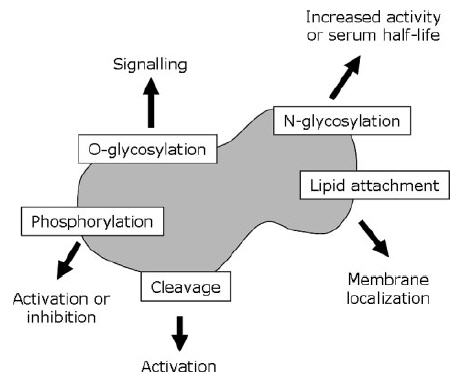
Glycosylation and Biopharmaceutical Manufacturing:
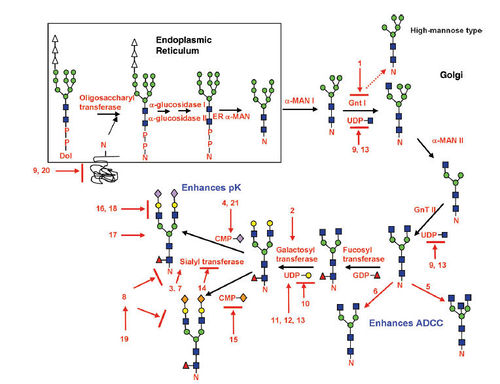
Protein glycosylation typically leads to a diverse array of product glycans due to multiple factors. Even with knowledge of human glycoform profiles for a particular glycoprotein, matching those attributes on the recombinant biologic is not trivial. Regulatory agencies around the world require biopharmaceutical companies to both characterize and maintain product quality attributes (including glycosylation) within defined acceptance limits. In biopharmaceutical manufacturing, the acceptance limits are based on what the cell line, upstream cell culture, harvest operation and downstream purification can support, and on the known glycoform species.
Current production processes usually use microbial systems, yeast or cell lines derived from insects (SF9), mice (SP20) or hamster (CHO). Glycosylation is notably sensitive to cell growth conditions. Changes in culture pH, the availability of precursors and nutrients, and the presence or absence of various cytokines and hormones can each affect the extent of glycosylation. In addition, the presence of sialidases and other glycosidases, either secreted or released by dead cells, can cause degradation of a previously intact product.
Control of this metabolic pathway can be manifested through a variety of different conditions, including the cell lines, bioprocess, and cell culture media used for protein production.Hossler P et al, Glycobiology,2009
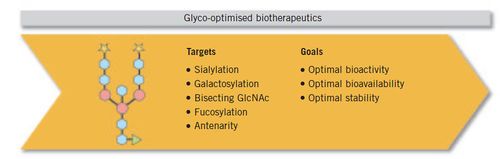
Glycosylation in Biosimilars:
A change is occurring in the biotechnologically derived drug industry with the impending arrival of biosimilars on the market. This is a challenge for biopharmaceutical companies where a cell line change, process changes and/or change of manufacturing site requires extensive comparability studies to prove that the molecule remains the same. Glycosylation has moved centre-stage in the development of biopharmaceutical drug products. Its influence on a protein’s characteristics has been acknowledged by regulatory requirements for the development of biosimilars that demand a high level of glycosylation-based similarity to the original product. As a consequence, glycosylated biosimilars need to be equipped with a similar pattern of glycosylation.Baumeister H et al, Glycotope
Relevant Patents' Example
| S.No | Patent/Publication No. | Date of Publication | Title | Abstract | Assignee | Problem | Solution |
| 1 | US6780607B2 | 8/24/2004 | Method for cell-free protein complete post-translational modification | The present invention relates to methods of production of the completely post-translationally modified protein by combination of cell-free protein synthesis and cell-free co-and post-translational modification. Previous cell-free protein synthesis system has only been capable of producing partially post-translationally modified protein but the present invention employs a co-and post-translational modification machinery that produces completely post-translationally modified protein. | DreamBiogen Co., Ltd. | For a mammalian protein to be active, it must show native post translational modifications. One way to produce proteins with native post translational modification is to culture the cells in native cell lines or modified eukaryotic hosts. This process is time consuming. Also, the available cell-free protein synthesis systems till then could not produce proteins with complete modifications. | An advanced cell free protein synthesis system is described which works by the combination of cell free protein synthesis and cell free complete post translational modification. The system comprises ribonucleotide triphosphates, sufficient amount of co- and post-translational modification machinery such as ER/golgiaparatus or ER/Golgi apparatus/plasma membrane, or other organelles and DNA or RNA template. |
| 2 | US6610516B1 | 8/26/2003 | Cell culture process | A glycoprotein is produced by a process comprising culturing mammalian host cells expressing nucleic acid encoding a glycoprotein in the presence of (a) a factor that modifies growth state in a cell culture, (b) a divalent metal cation that can adopt and prefers an octahedral coordination geometry, and/or (c) a plasma component. In this process, the occupancy of an N-linked glycosylation site occupied only in a fraction of a glycoprotein is enhanced. Such culturing is preferably carried out at a temperature of between about 30° C. and 35° C. and/or in the presence of up to about 2 mM of a butyrate salt and/or in the presence of a cell-cycle inhibitor. | Genentech, Inc. | There is a need for increasing glycosylation site occupancy in glycoproteins having multiple glycoforms to produce glycoprotein therapeutics of consistent product quality. | A few modifications to the cell culture conditions have been described to increase the occupancy of a glycosylation site in productions of glycoprotein with multiple glycoforms. The modifications include addition of butyrate salt or divalent cations such as iron and manganese. Also, the cell culture temperature has been reduced to 30 - 35 degrees C instead of traditional 37 degrees C. These modifications help control the cells in Go/G1 phase of cell cycle. Glycosylated Human t-PA has been produced. |
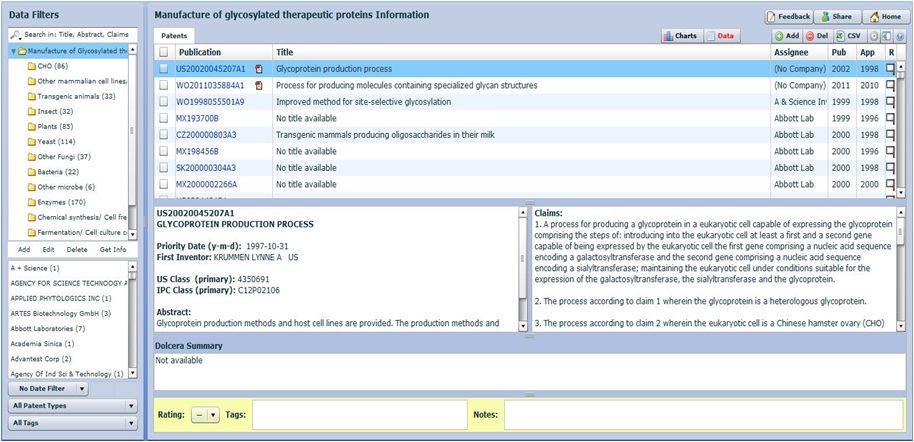
Click here to download analyzed samples
Taxonomy
Note: Taxonomy will be further populated as per detailed analysis
Assignee Analysis and IP Activity
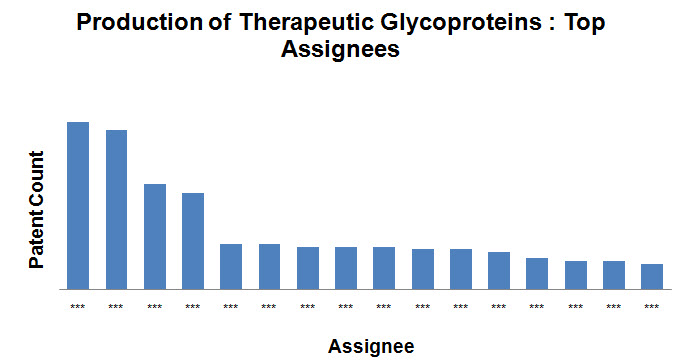
Top Assignees
- Roche Holding Ltd, Kirin Holdings Company Limited and Novo Nordisk are the top assignees in the field.
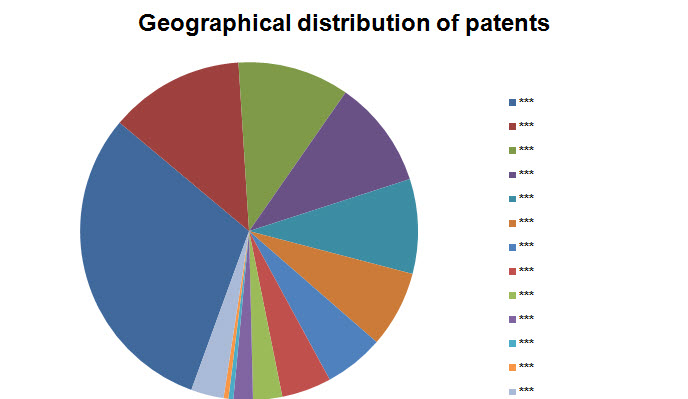
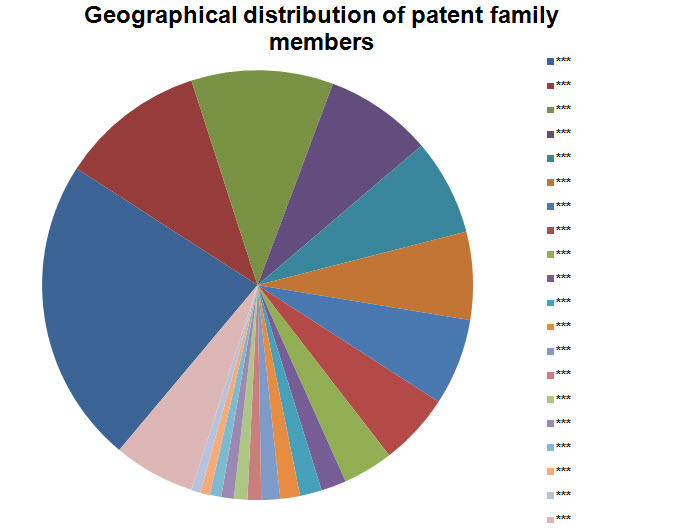
Geographical Distribution
- Based on Patent No.
Note: Countries with three or lesser patents have been grouped into Others.
- Including all family members
Note: Countries with 15 or lesser patents have been grouped into Others.
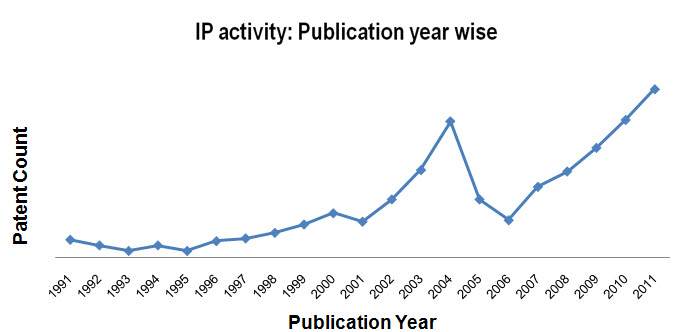
IP activity
- Patent activity based on Publication Year
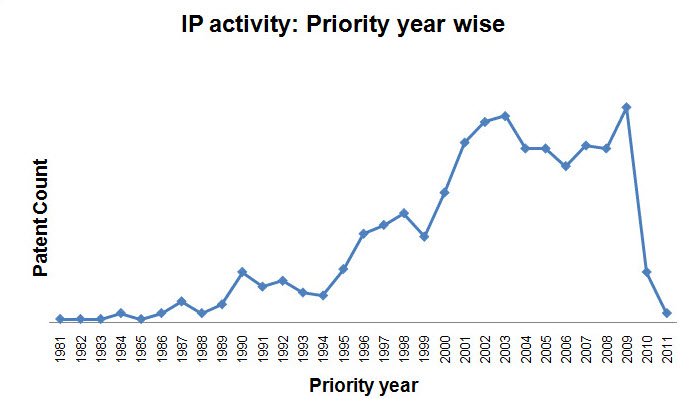
- Patent activity based on Priority Year
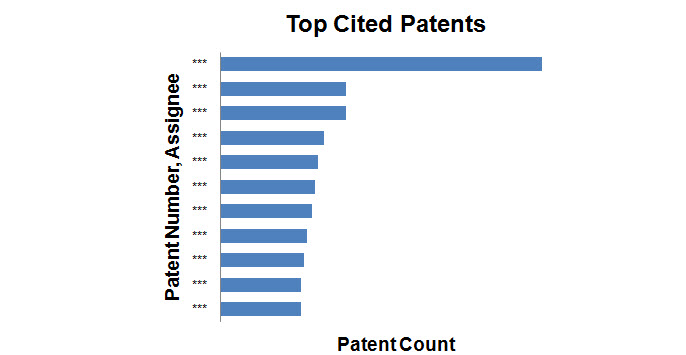
Top Cited patents
- Patents with the maximum number of forward citations were determined and the graph shows the top 10 patents with corresponding assignees.
Dashboard
Patents were categorized on the basis of patent focus- whether novel or modified cell lines or hosts, transgenic animals, novel enzymes, chemical/cell free methods of synthesis, and novel fermentation methods or culture conditions. Only those patents with method of manufacture of therapeutic glycoproteins as their focus have been considered in this analysis.
Click here to access dashboard
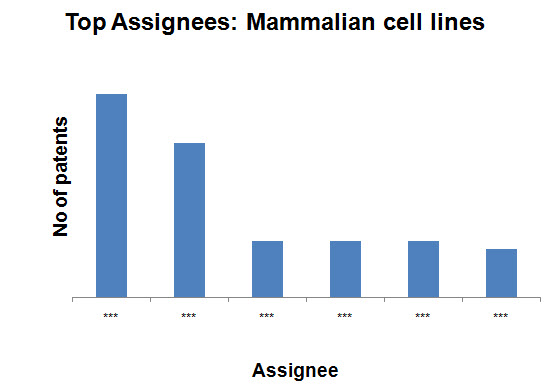
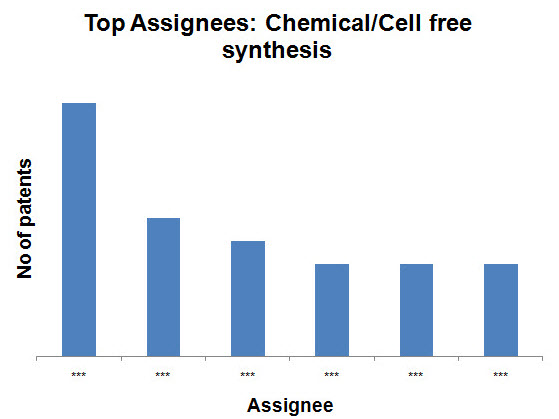
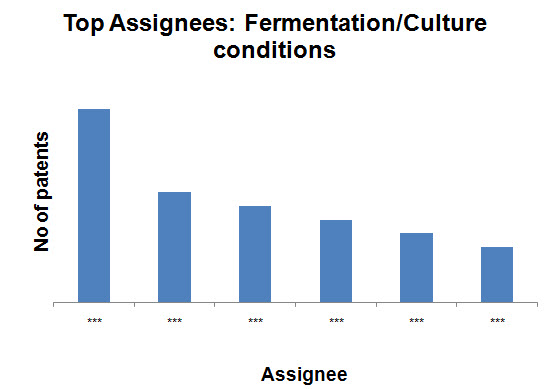
Top Assignees on the basis of production methods
- Mammalian cell lines
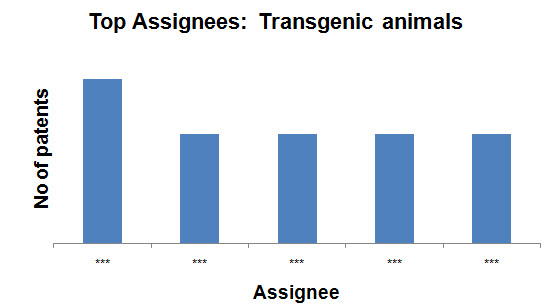
- Transgenic animals
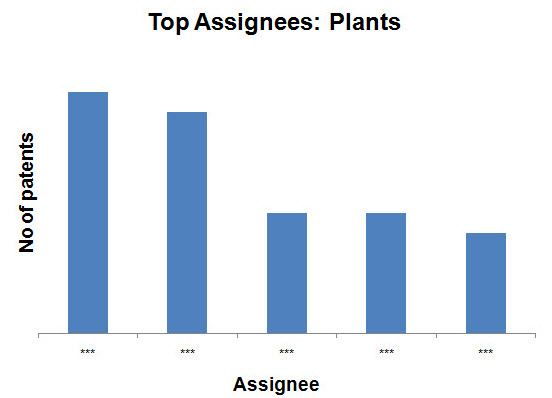
- Plants
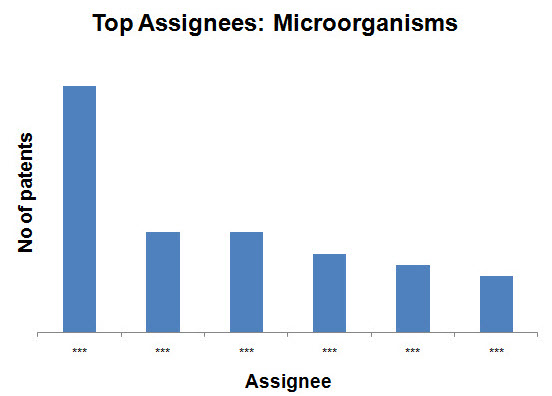
- Microorganisms
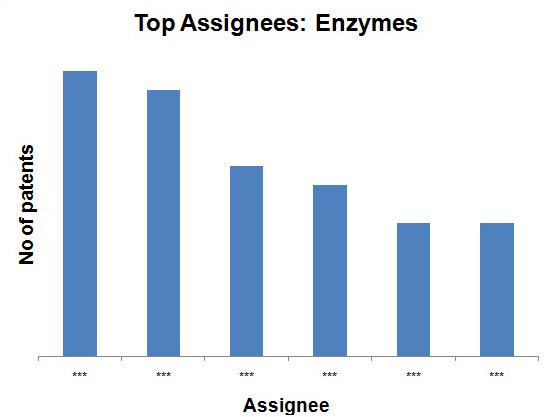
- Enzymes
- Chemical/Cell free synthesis
- Fermentation/Culture conditions
Patent product mapping example
| S.No. | Patent/ Pub No. | Title | Assignee/Applicant | Product | Product image |
| 1 | US5459031 | Methods for controlling sialic acid derivatives in recombinant glycoproteins | Amgen Inc. | Epogen® |
|
- Note: The patents focusing on methods to produce glycosylated proteins, methods to alter the glycosylation pattern of the proteins, cell lines for producing glycosylated proteins or altering the glycosylation pattern, enzymes for producing glycosylated proteins or altering the glycosylation pattern were considered on target in the analysis. Patents focusing on Purification of glycoproteins or therapeutic use of a glycoprotein were considered as off target.