Difference between revisions of "Interferon For Treatment of Melanoma"
Ankitbiyani (Talk | contribs) (→Sample Patents Analysis) |
Ankitbiyani (Talk | contribs) (→Geographical Distribution based on family members) |
||
| Line 710: | Line 710: | ||
* The geographical distribution is based on 10 sample patent numbers along with all their family members. | * The geographical distribution is based on 10 sample patent numbers along with all their family members. | ||
[[Image:Geographical Distribution based on Family members Melanoma 1.jpg|center|thumb|500 px| Geographical Distribution based on Family members Melanoma]] | [[Image:Geographical Distribution based on Family members Melanoma 1.jpg|center|thumb|500 px| Geographical Distribution based on Family members Melanoma]] | ||
| + | |||
| + | ==Market Report== | ||
| + | ===Interferon types & Their Compositions=== | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Generic Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Brand Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Company Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Composition'''</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Alpha IFN</center> | ||
| + | | style="padding:0.079cm;"| <center>Intron®,Roferon®-A</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Interferon alfa-2aInactive Ingredients- sodium chloride, ammonium acetate, polysorbate 80, glycine, sodium phosphate dibasic,sodium phosphate monobasic, human albumin, preservative: benzyl alcohol.</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''2'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Beta IFN</center> | ||
| + | | style="padding:0.079cm;"| <center>Avonex</center> | ||
| + | | style="padding:0.079cm;"| <center>Biogen IDEC</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Beta interferon,Inactive Ingredients-65 to 90 wt % of polyol,and a p-hydroxybenzoate,carboxymethyl cellulose,human serum albumin</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''3'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Gamma IFN</center> | ||
| + | | style="padding:0.079cm;"| <center>Actimmune®</center> | ||
| + | | style="padding:0.079cm;"| <center>Intermune</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-interferon gamma-1b.,Inactive Ingredients-Polyethylene Glycol,dextran ,hydroxyethylstarch </center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''4'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Pegylated IFN</center> | ||
| + | | style="padding:0.079cm;"| <center>Peg Intron</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering-Plough</center> | ||
| + | | style="padding:0.079cm;"| <center>Active ingredient-peginterferon alfa-2b,Inactive ingredients: dibasic sodium phosphate anhydrous, monobasicsodium phosphate dihydrate, sucrose, polysorbate 80.</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''5'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Recombinant IFN</center> | ||
| + | | style="padding:0.079cm;"| <center>(Rebetron®, Rebetol®).</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Ribavirin,Inactive Ingredients-microcrystalline cellulose, lactose monohydrate, croscarmellose sodium,sodium phosphate dibasic and sodium phosphate dibasic and sodium phosphate monobasic as buffering agents;human albumin as a stabilizer.</center> | ||
| + | |||
| + | |} | ||
| + | |||
| + | ===Interferon Types & Description Of Products=== | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Company Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Product'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Description'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Source'''</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>Intron®,Roferon®-A</center> | ||
| + | | style="padding:0.079cm;"| <center>Intron A is an interferon, a group of naturally occurring proteins that were first discovered as a result of their ability to prevent viral replication. Intron A is marketed in 72 countries worldwide for as many as 16 indications.In the United States it has been cleared for use by the FDA for chronic viral hepatitis B, chronic viral hepatitis C, malignant melanoma, hairy cell leukemia, AIDS-related Kaposi’s sarcoma and condylomata acuminata (venereal warts).INTRON A recombinant for Injection has been classified as an alpha interfero nand is a water-soluble protein with a molecular weight of 19,271 daltons produced by recombinant DNA techniques. It is obtained from the bacterial fermentation of a strain of Escherichia coli bearing a genetically engineered plasmid containing an interferon alfa- 2b gene from human leukocytes. The fermentation is carried out in a defined nutrient medium containing the antibiotic tetracycline hydrochloride at a concentration of 5 to 10 mg/L; the presence of this antibiotic is not detectable in the final product. The specific activity of interferon alfa-2b, recombinant is approximately 2.6 x 108 IU/mg protein as measured by the HPLC assay.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.introna.com/maintenance.html http://www.introna.com/maintenance.html]</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''2'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Biogen IDEC</center> | ||
| + | | style="padding:0.079cm;"| <center>Avonex</center> | ||
| + | | style="padding:0.079cm;"| <center>Avonex, manufactured by Biogen, is a form of beta interferon (interferon beta, IFN-b) used to modify the course of multiple sclerosis. While not a cure, Avonex has been shown in clinical trials to reduce the average relapse rate in people with the relapsing-remitting multiple sclerosis form of the disease. It is identical to the naturally occurring protein found in the human body. It is manufactured by extracting the drug from Chinese hamster ovary cells. Avonex is the same substance as Rebif but administered differently (30 mcg, intra-muscularly, once a week as against 22 mcg or 44 mcg, sub-cutaneously, 3 times a week for Rebif). Avonex is usually given in the large muscles of the thigh, upper arm, or hip.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.mult-sclerosis.org/Avonex.html http://www.mult-sclerosis.org/Avonex.html]</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''3'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Intermune</center> | ||
| + | | style="padding:0.079cm;"| <center>Actimmune®</center> | ||
| + | | style="padding:0.079cm;"| <center>Actimmune(R) is a synthesized version of interferon gamma, a naturally occurring protein believed to stimulate the immune system. InterMune markets Actimmune(R) for the treatment of two life-threatening congenital diseases: chronic granulomatous disease and severe, malignant osteopetrosis. The most common side effects are flu-like symptoms, including headache, fatigue, fever, chills, and rash. InterMune was granted two composition-of-matter patents related to interferon gamma-1b in the United States, extending its patent protection until 2022.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.actimmune.com/ http://www.actimmune.com/]</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''4'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering-Plough</center> | ||
| + | | style="padding:0.079cm;"| <center>Peg Intron</center> | ||
| + | | style="padding:0.079cm;"| <center>Peg-Intron (peginterferon alfa-2b) Powder for Injection has been approved by the FDA.Peg-Intron is a longer-acting formulation of Schering-Plough's Intron A, which is a recombinant version of a naturally occurring alpha interferon. In contrast to Intron A, which is administered three times weekly, Peg-Intron is administered subcutaneously once a week. This reduced frequency of administration may increase patient compliance.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.pegintron.com/index.html http://www.pegintron.com/index.html]</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''5'''</center> | ||
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>(Rebetron®, Rebetol®).</center> | ||
| + | | style="padding:0.079cm;"| <center>REBETOL is a medicine used with either interferon alfa-2b (Intron A) or peginterferon alfa-2b (PegIntron) to treat chronic (lasting a long time) hepatitis C infection in people 3 years and older with liver disease.REBETOL Capsules consist of a white powder in a white, opaque, gelatin capsule. Each capsule contains 200 mg ribavirin and the inactive ingredients microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The capsule shell consists of gelatin, sodium lauryl sulfate, silicon dioxide, and titanium dioxide. The capsule is printed with edible blue pharmaceutical ink which is made of shellac, anhydrous ethyl alcohol, isopropyl alcohol, n-butyl alcohol, propylene glycol, ammonium hydroxide, and FD&C Blue #2 aluminum lake.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.merck.com/product/home.html http://www.merck.com/product/home.html]</center> | ||
| + | |||
| + | |} | ||
| + | |||
| + | ===Global Revenue Data Of products=== | ||
| + | <table cellpadding="3" cellspacing="0"> | ||
| + | <tr> | ||
| + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>S.No</strong></td> | ||
| + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Generic Name</strong></td> | ||
| + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Brand Name</strong></td> | ||
| + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Company</strong></td> | ||
| + | <td colspan="3" rowspan="1" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Global revenue ($ Million )</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2008</strong></td> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2009</strong></td> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2010</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>1</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Alpha IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Intron®,Roferon®-A</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Schering Corporation</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="bottom">38.4</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Beta IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Avonex</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center"> Biogen IDEC</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">2518.4</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">2322.9</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);" align="center" valign="center">2202.6</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>3</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Gamma IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Actimmune®</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Intermune</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">29880</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">25428</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>4</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Pegylated IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Peg Intron</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center"> Schering-Plough</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">148.7</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>5</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Recombinant IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">(Rebetron®, Rebetol®).</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Schering Corporation</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">36.1</td> | ||
| + | <td style="border-width: thin thick thick thin; border-style: solid; border-color: rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | </table> | ||
Revision as of 00:52, 24 February 2011
Contents
- 1 Dashboard
- 2 Objective
- 3 Overview
- 4 Interactive Taxonomy
- 5 Concept Table
- 6 Taxonomy
- 7 Intellectual property
- 7.1 Search strategy and concept
- 7.2 Search in Micropatent full text - English language search
- 7.3 Search in Micropatent full text - Foreign language search
- 7.4 Search in Micropatent MPI-INPADOC - English language search
- 7.5 Search in Micropatent MPI-INPADOC - Foreign language search
- 7.6 Search in Japanese database
- 8 Sample patents
- 9 Patent Ranking
- 10 IP Activity Graphs Of Sample Patents
- 11 Market Report
Dashboard
- Click here to view Sample Dashboard
NOTE: You need to install Internet Explorer 8.0 and Adobe Flash Player to view the Dashboard.
Please download Internet Explorer 8.0 and Adobe Flash player
Objective
Primary objective of the study was to perform a prior art search on usage of interferon for the treatment of melanoma.
To achieve our objective we performed following steps:
- Created a multi level taxonomy to categorize the patents using interferon for melanoma treatment
- Marked out relevant IPC, ECLA, US classes and Japanese F-term available for technology in question.
- Identified and clubbed relevant keywords with classes to extract relevant patents.
- Checked for patents in US, EP, PCT, JP, Great Britain, and German patent records
- Performed MPI-INPADOC search which cover bibliographic data for 71 countries and legal status for 42 countries
- Analyzed the patents and prepared an IPmap covering relevant patents for client usage.
Overview
Interferon
Interferons (IFNs) are natural cell-signaling proteins produced by the cells of the immune system of most vertebrates in response to challenges such as viruses, parasites and tumor cells. They belong to the large class of glycoproteins known as cytokines and are produced by a wide variety of cells in response to the presence of double-stranded RNA, a key indicator of viral infection. Source
Interferons assist the immune response by inhibiting viral replication within host cells, activating natural killer cells and macrophages, increasing antigen presentation to T lymphocytes, and increasing the resistance of host cells to viral infection. There are 3 known classes of interferons; type I, type II and type III. All classes are very important in fighting viral infections. Recent studies have shown that Interferon can also help stop the growth and spread of cancer cells. Source
Melanoma
Melanoma is the most serious type of skin cancer. It begins in skin cells called melanocytes. Melanocytes are the cells that make melanin, which gives skin its color. Melanin also protects the deeper layers of the skin from the sun's harmful ultraviolet (UV) rays.When people spend time in the sunlight, the melanocytes make more melanin and cause the skin to tan. This also happens when skin is exposed to other forms of ultraviolet light (such as in a tanning booth). If the skin receives too much ultraviolet light, the melanocytes may begin to grow abnormally and become cancerous. This condition is called melanoma.People with melanoma who have one or more positive lymph nodes are at a high risk to have their melanoma recur. It is believed that 70 to 80% of these individuals will have their melanoma come back within the next three to five years. Source
Interferon for treatment of melanoma
Over the past several decades, the incidence of melanoma has increased at a faster rate than that of any other solid tumor. In the 1930s, the lifetime risk for a person living in the U.S. to develop melanoma was 1 in 1,500. Currently, that risk is 1 in 74, and for 2003 it was estimated that 51,400 cases of invasive melanoma would be diagnosed. While efforts to improve early diagnosis through education have resulted in the increased detection of early-stage melanoma, many patients still present with high-risk primary melanomas.
A beacon of hope in the treatment of melanoma has long been the observation that melanoma is susceptible to attack by the host’s immune system. This has resulted in the testing of a remarkably broad spectrum of immunotherapies, including the use of nonspecific immunostimulants, various approaches to vaccine therapies, and cytokine therapy. Many of these approaches failed to demonstrate a significant clinical impact, and the practitioner had been left with few options in treating high-risk melanoma patients with adjuvant therapy. One exception to this, however, has been the use of adjuvant interferon alpha (IFN-{alpha})
While the precise mechanism of action remains poorly understood, there are multiple antitumor effects of IFN-{alpha}. These include a direct antiproliferative effect, the enhancement of natural killer cell activity, and the upregulation of tumor antigens and/or HLA class I and class II antigens. Initial phase II clinical studies with IFN-{alpha} in metastatic melanoma showed response rates in the 10%–20% range [4, 5]. These response rates, while encouraging, were not significant enough to lead to its widespread use in the treatment of metastatic melanoma. Source
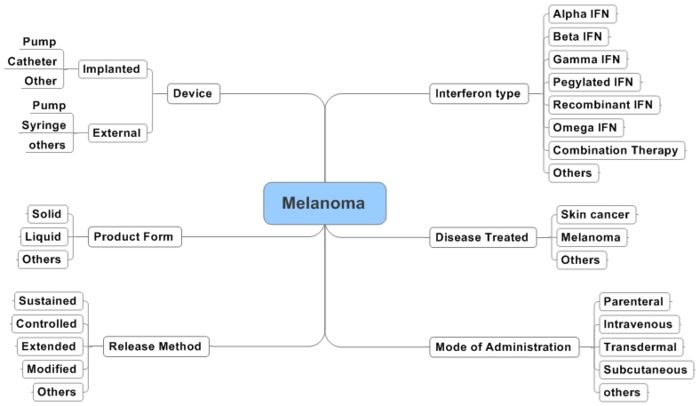
Interactive Taxonomy
Concept Table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
French Keywords Concept table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
German Keywords Concept Table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
Taxonomy
Class codes identified for searches
- Relevant IPC classes
| IPC | |||
| Sr. No. | Class Code | Class definition | Class coverage |
| 1 | A61K003819 | Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons | Broad |
| 2 | A61K003821 | Medicinal preparations containing peptides Interferon | Specific |
| 3 | C07K001452 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons | Broad |
| 4 | C07K014555 | Peptides having more than 20 amino acids - Interferon | Specific |
| 5 | C07K001456 | Peptides having more than 20 amino acids - IFN-alpha | Specific |
| 6 | C07K014565 | Peptides having more than 20 amino acids - IFN-beta | Specific |
| 7 | C07K001457 | Peptides having more than 20 amino acids - IFN-gamma | Specific |
| 8 | A61P003500 | Therapeutic activity of chemical compounds or medicinal preparations -antineoplastic agents | Broad |
- Relevant ECLA classes
| ECLA | |||
| Sr. No. | Class Code | Class definition | Class coverage |
| 1 | A61K003819 | Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons | Broad |
| 2 | A61K003821 | Medicinal preparations containing Interferon | Specific |
| 3 | A61K38/21A | Medicinal preparations containing IFN-alpha | Specific |
| 4 | A61K38/21B | Medicinal preparations containing IFN-beta | Specific |
| 5 | A61K38/21C | Medicinal preparations containing IFN-gamma | Specific |
| 6 | C07K001452 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons | Broad |
| 7 | C07K014555 | Peptides having more than 20 amino acids - Interferon | Specific |
| 8 | C07K001456 | Peptides having more than 20 amino acids - IFN-alpha | Specific |
| 9 | C07K014565 | Peptides having more than 20 amino acids - IFN-beta | Specific |
| 10 | C07K001457 | Peptides having more than 20 amino acids - IFN-gamma | Specific |
| 11 | C07K014715G | Receptors; Cell surface antigens; Cell surface determinants - for interferons - | Specific |
- Relevant US classes
| US class | ||
| Sr. No. | Class Code | Class definition |
| 1 | 4240854 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - this subclass provides for patents which broadly claim interferon or a method of treatment of interferon where the classification of the interferon as alpha, beta or gamma interferon is impossible |
| 2 | 4242811 | DRUG, BIO-AFFCTING AND BODY TREATING COMPOSITIONS - Virus (e.g., interferon-inducing virus, etc.) |
| 3 | 42400141 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - Attached to lymphokine, cytokine, or other secreted growth regulatory factor, differentiation factor, or intercellular mediator specific for a hematopoietic cell (e.g., interferon, interleukin, macrophage factor, colony stimulating factor, erythropoietin); derivative thereof |
| 4 | 514889 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - INTERFERON INDUCER |
| 5 | 530351 | CHEMISTRY: NATURAL RESINS OR DERIVATIVES; PEPTIDES OR PROTEINS; LIGNINS OR REACTION PRODUCTS THEREOF - Lymphokines, e.g., interferons, interlukins, etc. |
| 6 | 930142 | PEPTIDE OR PROTEIN SEQUENCE - Interferon |
| 7 | 4240851 | LYMPHOKINE - Included in this and the indented subclasses interferon, interleukin and macrophage factors (monokines) |
| 8 | 4240855 | Gamma or immune: This subclass is indented under subclass 85.4. Subject matter in which the interferon is gamma or immune interferon. |
| 9 | 4240856 | Subject matter in which the interferon is beta or fibroblast interferon. |
| 10 | 4240857 | Subject matter in which the interferon is alpha or leukocyte interferon. |
Intellectual property
Search strategy and concept
Date of Search: 1836 to October 26, 2009 Database used: Micropatent - Include extensive full text and MPI-Inpadoc searches
Search in Micropatent full text - English language search
Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted.
| Sr. No. | Search concept | Search Scope | Search reason | Class Code (IPC,US,ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | Title, Abstract and Claims | Specific classes of interferon AND melanoma keywords | A61K003821* OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G OR 4240854 OR 4242811 OR 42400141 OR 514889 OR 530351 OR 930142 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 576 |
| 2 | Interferon for treating Melanoma | Title, Abstract and Claims | Broad classes of interferon AND melanoma, interferon keywords | A61K003819 OR C07K001452 OR 4240851 OR 4240855 OR 4240856 OR 4240857 OR A61P003500 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 756 |
| 3 | Final query | 1 OR 2 | 1019 records 571 unique records | |||
Search in Micropatent full text - Foreign language search
Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted.
| Sr. No. | Search concept | Language | Search Scope | Search reason | Class Code (IPC, ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | French | Title, Abstract and Claims | Specific classes of interferon AND melanoma’s foregin langugae keywords | A61K003821* OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 184 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 2 | Interferon for treating Melanoma | French | Title, Abstract and Claims | Broad classes of interferon AND melanoma’s and interferon’s foregin langugae keywords | A61K003819 OR C07K001452 OR A61P003500 | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 3375 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 3 | Final query | 1 OR 2 | 3422 hits (2023 unique records, 30-35 % relevant) | ||||
Search in Micropatent MPI-INPADOC - English language search
Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract.
| Sr. No. | Search concept | Search Scope | Search reason | Class search | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | Title and Abstract | Specific IPC classes of interferon AND melanoma keywords | A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 174 |
| 2 | Interferon for treating Melanoma | Title and Abstract | Broad IPC classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 484 |
| 3 | Interferon for treating Melanoma | Title and Abstract | Specific ECLA classes of interferon AND melanoma keywords | A61K03821* OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 OR C07K014715G | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 102 |
| 4 | Interferon for treating Melanoma | Title and Abstract | Broad ECLA classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 9 |
| 5 | Final query | 1 OR 2 OR 3 OR 4 | 587 hits 232 unique records | |||
Search in Micropatent MPI-INPADOC - Foreign language search
Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract.
| Sr. No. | Search concept | Language | Search Scope | Search reason | Class Code (IPC, ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | French | Title and Abstract | Specific IPC/ECLA classes of interferon AND melanoma keywords | A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 4 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 2 | Interferon for treating Melanoma | French | Title and Abstract | Broad IPC classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR Interféron* OR *Interféron OR huIFN) AND (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 25 hits |
| German | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN AND (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 3 | Final query | 1 OR 2 | 29 hits | ||||
Search in Japanese database
Database: IPDL (Industrial property digital library), Japan
Date of search: 1900/01/01 to 2009/10/26
Total patents: 846 (Relevancy ~10%)
- F-Terms and theme used in search
| Japanese F-term search | Definition | ||
| Sr. No. | F- Term theme | 4H045 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof |
| 1 | F-term | DA15 | Peptide or protein characterised by function - Interferons |
| 2 | F-term | DA16 | Alpha-interferons |
| 3 | F-term | DA17 | Beta-interferons |
| 4 | F-term | DA18 | Gamma-interferons |
Sample patents
| S.No. | Patent/Publication No. | Date of Publication | Assignee / Applicant | Inventor(s) | Title | Dolcera Summary |
| 1 | US7482014B2 | 1/27/2009 | Schering Corporation | Rybak, Mary Ellen and Rose, Esther Helen | Melanoma therapy | The invention relates to a method of treating a patient having Stage IIB or Stage III melanoma which has been surgically removed. A first dose of 6.0 micrograms/kg of pegylated interferon alpha-2b once a week for eight weeks, and then administering to the patient a second dose of 3.0 or less micrograms/kg of pegylated interferon alpha-2b once a week for the remainder of a five year treatment period. |
| 2 | US5997858A | 12/7/1999 | Pharma Pacific Pty Ltd. | Tovey, Michael Gerard and Kaido, Thomas James | Stimulation of host defense mechanisms against tumors | A method for treating multiple myeloma, hairy cell leukemia, malignant melanoma, brain tumors etc. by administering a therapeutically effective amount of an interferon (1500 IU to about 20×106 IU for a 70 kg man per day) via oromucosal contact. |
| 3 | EP288055A1 | 10/26/1988 | MERRELL DOW PHARMACEUTICALS INC. | Sunkara, Sai P. | Use of ODC inhibitors, dacarbazine, and interferon, in the treatment of malignant melanoma | A combinational therapy containing an ornithine decarboxylase inhibitor, Interferon and Dacarbazine for simultaneous, separate or sequential use in treating rapidly-proliferating cell-growth disease such as melanoma. |
| 4 | EP241242A1 | 10/14/1987 | CETUS ONCOLOGY CORPORATION | Rudolph, Alfred | The use of interferon-beta and interleukin-2 for combination therapy and compositions therefor | A composition comprising of a mixture of lFN- beta and IL-2 for administration to human patients for therapeutic or prophylactic treatment of cancer such as colon cancer, melanoma, renal cell cancer, lung cancer. |
Sample Patents Analysis
- The above sample patents were analysed according to the taxonomy.
Click here to download Sample analysis sheet on Interferon for treatment of Melanomas
Patent Ranking
10 Sample Patents were ranked according to the patent focus.
- Patent Ranking Details
1 : Granted Patent & Focus in Independent Claim
2 : Granted Patent & Focus in Dependent Claim
3 : Published Patent & Focus in independent Claim
4 : Published Patent & Focus in Dependent claim
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
IP Activity Graphs Of Sample Patents
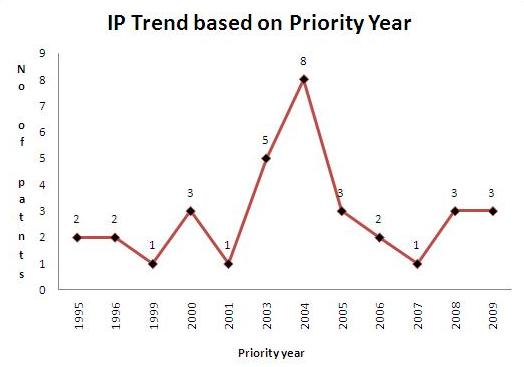
IP activity based on priority years
- Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on priority years.
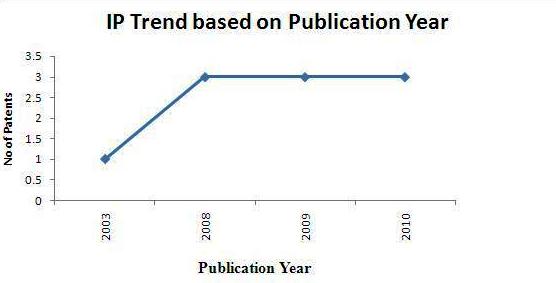
IP activity based on publication years
- Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on publication years.
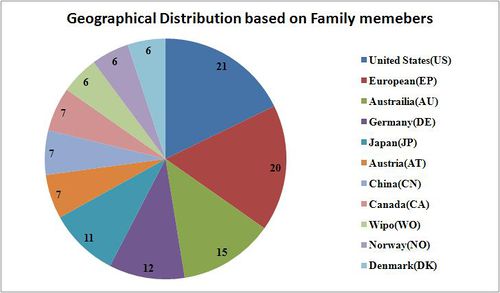
Geographical Distribution based on family members
- The geographical distribution is based on 10 sample patent numbers along with all their family members.
Market Report
Interferon types & Their Compositions
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
Interferon Types & Description Of Products
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
Global Revenue Data Of products
| S.No | Generic Name | Brand Name | Company | Global revenue ($ Million ) | ||
| 2008 | 2009 | 2010 | ||||
| 1 | Alpha IFN | Intron®,Roferon®-A | Schering Corporation | 38.4 | ||
| 2 | Beta IFN | Avonex | Biogen IDEC | 2518.4 | 2322.9 | 2202.6 |
| 3 | Gamma IFN | Actimmune® | Intermune | 29880 | 25428 | |
| 4 | Pegylated IFN | Peg Intron | Schering-Plough | 148.7 | ||
| 5 | Recombinant IFN | (Rebetron®, Rebetol®). | Schering Corporation | 36.1 | ||