Difference between revisions of "Clinical Trial Database"
From DolceraWiki
(→Clinical Trials databases covered) |
(→Clinical Trials data sources covered) |
||
| Line 5: | Line 5: | ||
*Dolcera has developed a clinical trial product pipeline database with a worldwide coverage of clinical trials done by the Medical device and pharmaceutical Industry. | *Dolcera has developed a clinical trial product pipeline database with a worldwide coverage of clinical trials done by the Medical device and pharmaceutical Industry. | ||
| − | ==Clinical | + | ==Clinical trials data sources covered== |
The database covers most countries of the world in the region of North and South America, Europe, Asia, and Australia. The sources of the databases are as under: | The database covers most countries of the world in the region of North and South America, Europe, Asia, and Australia. The sources of the databases are as under: | ||
* '''Government Registries''': The Government Registries of many countries in the world gives us a significant amount of data regarding the clinical Trials that is carried on in their respective countries like [http://www.clinicaltrials.gov Clinicaltrials.gov]. Apart from Clinical trials registries of each country, Dolcera has also identified other key Government sites which holds a set of registries of a country like the [http://apps.who.int/trialsearch/ WHO clinical trial registry]. | * '''Government Registries''': The Government Registries of many countries in the world gives us a significant amount of data regarding the clinical Trials that is carried on in their respective countries like [http://www.clinicaltrials.gov Clinicaltrials.gov]. Apart from Clinical trials registries of each country, Dolcera has also identified other key Government sites which holds a set of registries of a country like the [http://apps.who.int/trialsearch/ WHO clinical trial registry]. | ||
Latest revision as of 12:51, 15 June 2010
Contents
[hide]Introduction
- Clinical trials are conducted to allow safety and efficacy data to be collected for new drugs or devices. These trials can only take place once satisfactory information has been gathered on the quality of the product and its non-clinical safety, and Health Authority/Ethics Committee approval is granted in the country where the trial is taking place.
- Depending on the type of product and the stage of its development, investigators enroll healthy volunteers and/or patients into small pilot studies initially, followed by larger scale studies in patients that often compare the new product with the currently prescribed treatment. As positive safety and efficacy data are gathered, the number of patients is typically increased. Clinical trials can vary in size from a single center in one country to multicenter trials in multiple countries.
- Due to the sizable cost a full series of clinical trials may incur, the burden of paying for all the necessary people and services is usually borne by the sponsor who may be a governmental organization, a pharmaceutical, or biotechnology company. Since the diversity of roles may exceed resources of the sponsor, often a clinical trial is managed by an outsourced partner such as a contract research organization.
- Dolcera has developed a clinical trial product pipeline database with a worldwide coverage of clinical trials done by the Medical device and pharmaceutical Industry.
Clinical trials data sources covered
The database covers most countries of the world in the region of North and South America, Europe, Asia, and Australia. The sources of the databases are as under:
- Government Registries: The Government Registries of many countries in the world gives us a significant amount of data regarding the clinical Trials that is carried on in their respective countries like Clinicaltrials.gov. Apart from Clinical trials registries of each country, Dolcera has also identified other key Government sites which holds a set of registries of a country like the WHO clinical trial registry.
- Meta Sites: Apart from the government registries, we cover meta sites which do provide comprehensive and vast amount of data. Some of these sites include Centerwatch, Controlled Clinical Trials.
- Pharma Association and Pharma Companies' Clinical Trial Listings: We use the IFPMA site and also the clinical trial information included on many of the pharmaceutical companies' websites.
- Financial Filings: We also mine companies' SEC filings and press releases for clinical trial information.
- Other Online Sources: Apart from the sources listed above, we also cover journals, news, clinical trial specific websites (e.g. in case of clinical trials for cancer, we have sources like the National Cancer Institute's clinical trials list).
Results
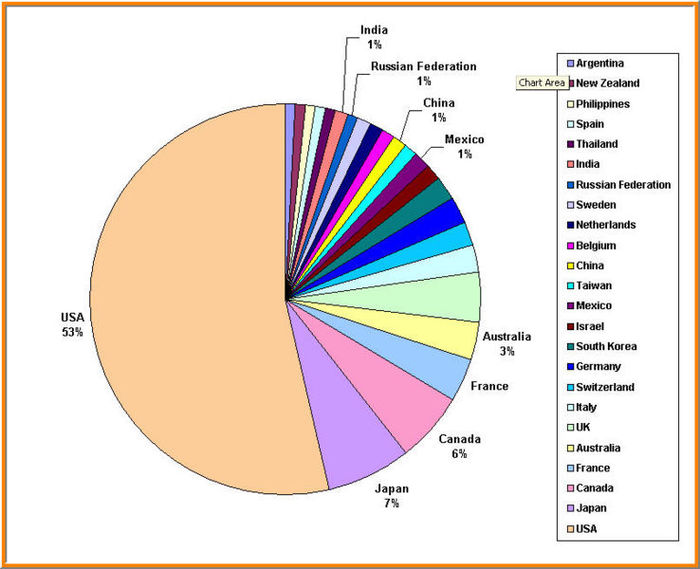
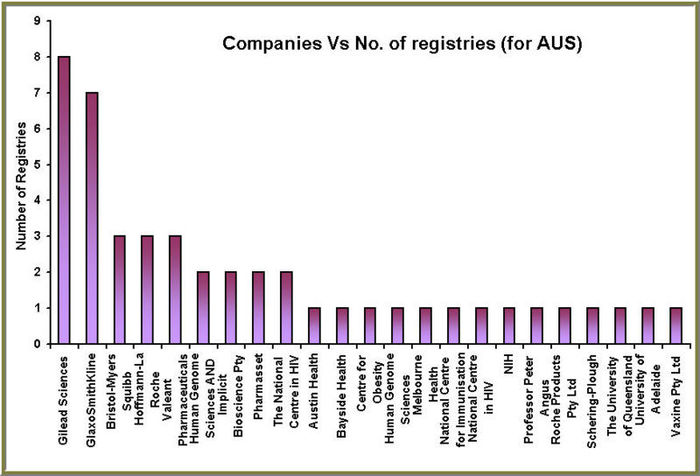
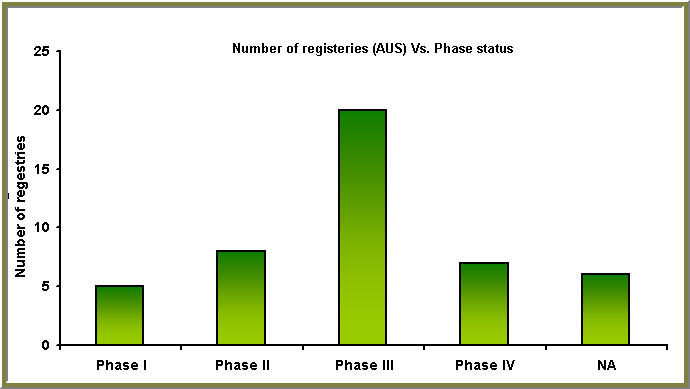
We took a case of finding Clinical trials for Hepatitis C and making a product pipeline database through the results that we obtained.We give a sample of this for the case of Clinical trials conducting in Australia for the time span of 1970-2007
Hepatitis C: Australian case study
Phase status Vs. No. of clinical trials
Click here to download the file with clinical trial entries for the Australian case study
We will soon come up with a comprehensive database on Clinical trials for user friendly interaction using our proprietary tool the Dolcera Dynamic Dashboard.
| Contact Dolcera |
|---|
| Email: info@dolcera.com |
| Phone: +1-650-269-7952, +91-40-2355-3493 |