Difference between pages "Hybrid Electric Vehicle Battery System" and "Bio-PET"
(→Introduction) |
(→Dashboard link) |
||
| Line 1: | Line 1: | ||
| − | + | PET (Polyethyeleneterephthalate) is used in a variety of applications, especially in the making of durable bottles. There is a significant increase in interest of late in producing PET from renewable sources. | |
| − | + | ||
| − | + | ||
| − | This report describes the | + | This report describes the various techniques being used in the production of PET from sources such as cellulose. The report covers: |
| + | # Various pathways for production of PET from renewable sources | ||
| + | # All the catalysts (e.g. Hydrogen, Sodium, etc.) and techniques (e.g. catalytic dehydration) used in production of each of the intermediate compounds (e.g. ethylene glycol) | ||
| + | # Search strategies for patents for the various pathways and methods for production of ethylene glycol and dimethyl terephthalate, which are then used to produce PET | ||
| + | # Classification and in-depth analysis of the patents | ||
| + | # Presentation of results of the patent analysis on the Dolcera dashboard | ||
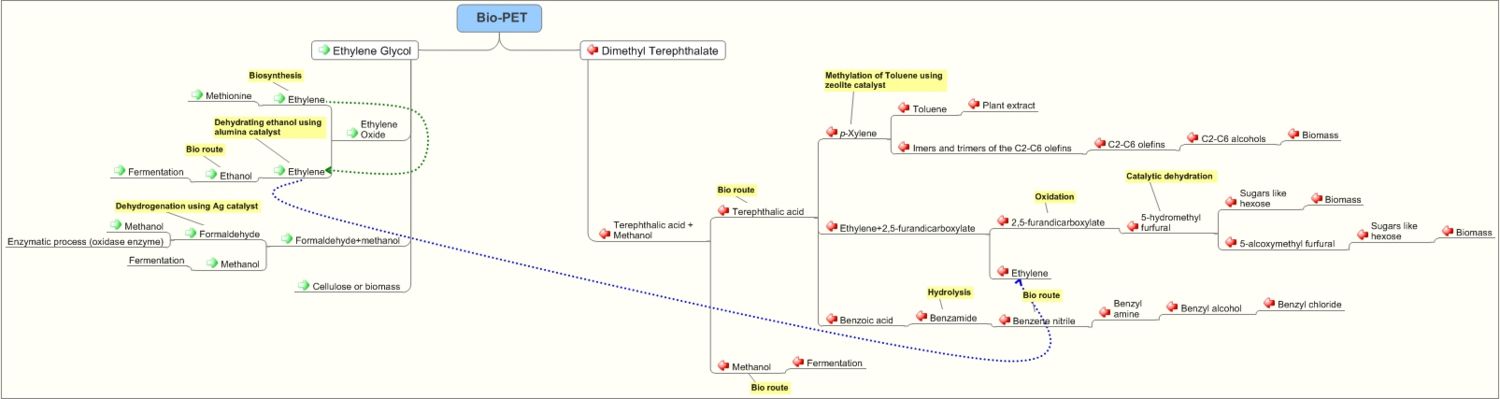
| − | == | + | =Pathways for production of Polyethyeleneterephthalate= |
| − | [[ | + | [[Image:bio pet1.jpg|thumb|center|1500px|'''Pathways for preparation of PET''']] |
| + | ==Polyethylene terephthalate== | ||
| + | [[Image:PET.jpg|thumb|center|350px]] | ||
| + | *Polyethylene terephthalate'''( (C'''<sub>'''10'''</sub>'''H'''<sub>'''8'''</sub>'''O'''<sub>'''4'''</sub>''')'''<sub>'''n'''</sub>''')''' (sometimes written poly(ethylene terephthalate)), is also abbreviated as PET, PETE, or PETP or PET-P) | ||
| + | *Some of the trade names of PET products are Dacron, Diolen, Tergal, Terylene, and Trevira fibers, Cleartuf, Eastman PET and Polyclear bottle resins, Hostaphan, Melinex, and Mylar films, and Arnite, Ertalyte, Impet, Rynite and Valox injection molding resins.<br> | ||
| + | *The materials that can be prepared using PET are bottles, tapes, films or pulled into fibers that are pressed into meshes or woven into fabrics. | ||
| + | ===Preparation of Polyethyleneterephthalate=== | ||
| + | * Polyethyleneterephthalate is prepared by reacting Dimethylterephthalate with Ethylene glycol in the presence of a catalyst. Dimethylterephthalate is obtained by treating Terephthalic acid with methanol. The catalyst is a salt of a metal like calcium, barium, cadmium, cobalt, lead, manganese, tin, zinc and chromium and a material selected from the group consisting of hydroxybenzophenone carboxylic acids and hydroxyacetophenone carboxylic acids. [[Media:polyethyleneterephthalate1.pdf|Source]]<br> | ||
| + | '''(Terephthalic acid) + 2CH<sub>3</sub>OH → (Dimethylterephthalate)''' | ||
| − | + | '''(Dimethylterephthalate) + CH<sub>2</sub>(OH)CH<sub>2</sub>(OH) + Catalyst → (Polyethyleneterephthalate)''' | |
| − | + | ||
| − | + | ||
| − | == | + | ==Ethylene glycol== |
| + | [[Image:Ethylene glycol.jpg|thumb|center|250px|Ethylene glycol]] | ||
| + | *Ethylene glycol'''(C'''<sub>'''2'''</sub>'''H'''<sub>'''4'''</sub>'''(OH)'''<sub>'''2'''</sub>''')''', (monoethylene glycol (MEG), 1,2-ethanediol, IUPAC name: ethane-1,2- diol,) | ||
| + | *Ethylene glycol is an odorless, colorless, syrupy, sweet tasting, toxic liquid. | ||
| + | *It is used as a heat transfer chemical i.e., as a coolant. Due to its low freezing point it is used as a deicing fluid for windshields and aircraft. | ||
| + | *Ethylene glycol is widely used to inhibit the formation of natural gas clathrates in long multiphase pipelines. Then ethylene glycol can be recovered from the natural gas and reused as an inhibitor after purification treatment that removes water and inorganic salts. | ||
| + | *Ethylene glycol has become increasingly important in the plastics industry for the manufacture of polyester fibers and resins, including polyethylene terephthalate, which is used to make plastic bottles for soft drinks. The antifreeze capabilities of ethylene glycol have made it an important component of vitrification mixtures for low-temperature preservation of biological tissues and organs. | ||
| + | *Ethylene glycol may also be used as a protecting group for carbonyl groups in organic synthesis. | ||
| + | *Ethylene glycol's high boiling point and affinity for water makes it an ideal desiccant for natural gas production. | ||
| + | [http://en.wikipedia.org/wiki/Ethylene_glycol Source] | ||
| − | |||
| − | |||
| − | + | ===Preparation of Ethylene Glycol=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Ethylene glycol is prepared in the following ways: | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''1.''' From ethylene: Ethylene is oxidized to ethylene oxide. Ethylene oxide upon hydrolysis ethylene glycol is prepared. | |
| − | + | ||
| − | ''' | + | '''7 H<sub>2</sub>C=CH<sub>2</sub> <nowiki>+</nowiki> 6 O<sub>2</sub> → 6 C<sub>2</sub>H<sub>4</sub>O <nowiki>+</nowiki> 2 CO<sub>2</sub> <nowiki>+</nowiki> 2 H<sub>2</sub>O''' |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''C<sub>2</sub>H<sub>4</sub>O <nowiki>+</nowiki> H<sub>2</sub>O → HOCH<sub>2</sub>CH<sub>2</sub>OH''' | |
| − | + | ||
| − | + | '''2.''' Ethylene glycol is prepared by reacting formaldehyde with methanol in the presence of organic peroxide(ditertiarybutylperoxide) [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4337371.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''CH<sub>3</sub>OH <nowiki>+</nowiki> HCHO <nowiki>+</nowiki> (CH<sub>3</sub>)<sub>3</sub>OOC(CH<sub>3</sub>)<sub>3</sub>→ CH<sub>2</sub>(OH)CH<sub>2</sub>(OH)''' | |
| − | + | '''Note:''' In the above two preparation methods for ethylene glycol we have used ethylene, methanol, formaldehyde. The following are their preparations: | |
| − | + | '''Ethylene preparation''': | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | 1. Ethylene is prepared biologically from methionine by using enzymes.[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6294716.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | [[Image:Ethylene biosynthesis.jpg|thumb|center|450px|Ethylene biosynthesis]]<br> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | 2. Ethanol upon dehydrating in the presence of catalyst ethylene is prepared.[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4134926.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | '''C<sub>2</sub>H<sub>5</sub>OH <nowiki>+</nowiki> Al<sub>2</sub>O<sub>3</sub>(Catalyst) → H<sub>2</sub>C=CH<sub>2</sub> <nowiki>+</nowiki> H<sub>2</sub>O''' | |
| − | + | '''Methanol preparation''': Biological process | |
| − | + | ||
| − | + | Methanol is prepared through fermentation process. Vegetable materials are fermented anerobically in the presence of ''Zymomonas mobilis'' bacteria. In this process ethanol and methanol are the products. Methanol is separated from ethanol by distillation. [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5070016.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | ||
| − | + | ||
| − | + | '''Formaldehyde preparation''': | |
| − | + | 1. Formaldehyde is prepared enzymatically. In this process the lower alkyl alcohols are converted into lower aldehydes by using oxidase enzymes. [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5234827.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | ||
| − | + | 2. Formaldehyde is prepared by dehydrogenation of methanol using a catalyst. The catalyst is silver or silver/gold alloy on an inert hard, nonporous support. [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4474996.PN.&OS=PN/4134926&RS=PN/4134926 Source] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''CH<sub>3</sub>OH <nowiki>+</nowiki> Ag (or Ag/Au alloy)(Catalyst) → HCHO <nowiki>+</nowiki> H<sub>2</sub>''' | |
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''Ethanol preparation''': | |
| − | + | ||
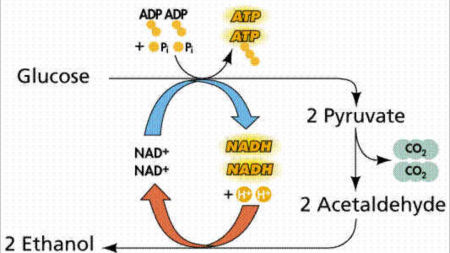
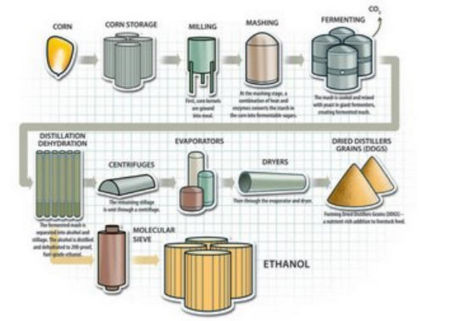
| − | + | Ethanol is prepared by fermentation process. | |
| − | + | ||
| − | + | ||
| − | + | [[Image:Ethanol2.jpg|thumb|center|450px|Ethanol - fermentation]] | |
| − | + | ||
| − | + | ||
| − | + | [[Image:Ethanol1.jpg|thumb|center|450px|Ethanol through Fermentation]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
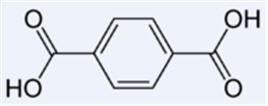
| − | === | + | ==Terephthalic acid== |
| − | [[Image: | + | [[Image:Terephthalic acid.jpg|thumb|center|350px|Terephthalic acid]] |
| + | *Terephthalic acid'''(C'''<sub>'''6'''</sub>'''H'''<sub>'''4'''</sub>'''(CO'''<sub>'''2'''</sub>'''H)'''<sub>'''2'''</sub>''')''' is a colourless solid, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually. It is one of three isomeric phthalic acids.<br> | ||
| + | *Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET) [http://en.wikipedia.org/wiki/Terephthalic_acid Source] | ||
| + | ===Preparation of Terephthalic acid=== | ||
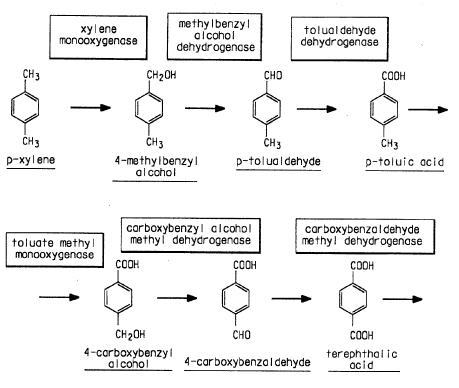
| + | * Biological preparation of Terephthalic acid [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6187569.PN.&OS=PN/4134926&RS=PN/4134926 Source] | ||
| + | [[Image:Terephthalic acid23.jpg|thumb|center|750px|Biological preparation of Terephthalic Acid]] | ||
| + | '''Preparation of ''p''-Xylene''': | ||
| − | + | ''p''-Xylene is prepared from toluene in the presence of a catalyst. This is a methylation reaction. The methylating agents are methanol, methyl chloride, methyl bromide, dimethylether or dimethylsulfide. The catalyst used is ZSM-23 zeolite material. Here this catalyst is used for attacking the methyl group in the para position of the toluene. [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4049738.PN.&OS=PN/4134926&RS=PN/4134926 Source]<br> | |
| − | + | ||
| − | + | ||
| − | + | '''Toluene + Methylating agent + ZSM-23 Zeolite(Catalyst)→ P-xylene''' | |
| − | + | '''Preparation of Toluene''':<br> | |
| + | 1. Toluene is an aromatic extract from the tropical colombian tree ''Myroxylon Balsamum'' [http://en.wikipedia.org/wiki/Toluene Source]<br> | ||
| + | 2. Toluene is prepared from wastes like old trees, cable wastes, polyethylene waster and polypropylene wastes. In this process the waste products are pulverised and heated to a temperature range of 150˚C to 500˚C at a pressure of 20 to 300 bar [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4642401.PN.&OS=PN/4134926&RS=PN/4134926 Source] | ||
| − | + | '''Note'''-Today most of the ethylene manufacturers are producing ethylene through petroleum cracking. | |
| − | We | + | ==Search Strategy== |
| − | + | * Database - Micropat | |
| + | * We conducted search on concepts that have a biological method of preparation only. | ||
| + | * We have not conducted search on production of ethanol (from which we prepare ethylene) since that is not the focus of this project. | ||
| + | ------------- | ||
| + | * '''NOTE''': Explanation of Query Concepts | ||
| + | * Query 1 - All Biological Process | ||
| − | + | * Query 2 - Ethanol Fermentation | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | * | + | * Final Query - Query 1 + Query 2 |
| + | ----------------- | ||
| − | + | Issue/Publication Years: 1836 - Date (9th June 2009) | |
| − | * ''' | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" |
| + | |align = "center" bgcolor = "#99CCFF"|'''S.No''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search string''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Hits''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFFF" colspan = "5"|'''Query 1''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''1''' | ||
| + | |align = "center" rowspan = "4"|'''Bio-Production of Terepthalic acid from Xylene''' | ||
| + | |Full patent spec. | ||
| + | |(((<nowiki>*</nowiki>4Benzenecarboxylic OR <nowiki>*</nowiki>4Benzenedicarboxylic OR Benzene<nowiki>*</nowiki>2para<nowiki>*</nowiki>2dicarboxylic OR benzene<nowiki>*</nowiki>5dicarboxylic OR Benzene<nowiki>*</nowiki>p<nowiki>*</nowiki>dicarboxylic OR p<nowiki>*</nowiki>Benzenedicarboxylic OR p<nowiki>*</nowiki>Carboxybenzoic OR p<nowiki>*</nowiki>Phthalic OR Para<nowiki>*</nowiki>Phthalic OR Terephthalic) ADJ acid) OR Acide<nowiki>*</nowiki>2terephtalique OR Kyselina<nowiki>*</nowiki>2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | ||
| + | |align = "center"| 102948 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''2''' | ||
| + | |Claims, Title or Abstract | ||
| + | |((bio<nowiki>*</nowiki> OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur<nowiki>*</nowiki>3 OR transformation OR transform OR conversion OR cataly<nowiki>*</nowiki>3 OR <nowiki>*</nowiki>synthe<nowiki>*</nowiki>)) | ||
| + | |align = "center"|360711 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''3''' | ||
| + | |Full patent spec. | ||
| + | | "1,4-dimethylbenzene" OR "p-methyltoluene" OR "1,4-xylene" OR <nowiki>*</nowiki>5dimethylbenzene OR <nowiki>*</nowiki>5xylene OR <nowiki>*</nowiki>3methyltoluene | ||
| + | |align = "center"|507183 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''4''' | ||
| + | |Combined Query | ||
| + | |align = "center"|1 AND 2 AND 3 | ||
| + | |align = "center"|25'''(20 Unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''5''' | ||
| + | |align = "center" rowspan = "6"|'''Production of Ethylene from Ethanol''' | ||
| + | |Claims, Title or Abstract | ||
| + | |Ethanol OR (ethyl ADJ1 alcohol) OR (grain ADJ1 alcohol) | ||
| + | |align = "center"|134288 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''6''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene<nowiki>*</nowiki> OR ((C2 OR "C.sub.2") NEAR5 olefins)) | ||
| + | |align = "center"|410332 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''7''' | ||
| + | |Claims, Title or Abstract | ||
| + | |produc<nowiki>*</nowiki>5 OR conver<nowiki>*</nowiki>5 OR manufactur<nowiki>*</nowiki>3 OR synthes<nowiki>*</nowiki>6 OR process OR method | ||
| + | |align = "center"|13960381 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''8''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(dehydrat<nowiki>*</nowiki> OR (water ADJ1 removal) OR hypohydrat<nowiki>*</nowiki> OR cataly<nowiki>*</nowiki> OR zeolite<nowiki>*</nowiki> OR silica OR alumina OR (trifluoromethanesulfonic ADJ1 acid) OR evaporat<nowiki>*</nowiki> OR desiccation OR drying OR trifluoromethanesulfonicacid OR (water NEAR5 remov<nowiki>*</nowiki>)) | ||
| + | |align = "center"|1643133 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''9''' | ||
| + | |Any Classification | ||
| + | |align = "center"|C07C OR B01D | ||
| + | |align = "center"|1267051 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''10''' | ||
| + | |Combined Query | ||
| + | |align = "center"|5 AND 6 AND 7 AND 8 AND 9 | ||
| + | |align = "center"|2606''' (1863 Unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''11''' | ||
| + | |align = "center" rowspan = "5"|'''Production of Ethylene through Biological process''' | ||
| + | |Claims, Title or Abstract | ||
| + | |((bio OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur<nowiki>*</nowiki>3 OR transformation OR transform OR conversation OR cataly<nowiki>*</nowiki>3)) OR ferment<nowiki>*</nowiki> OR microorganism<nowiki>*</nowiki> OR microb<nowiki>*</nowiki> OR <nowiki>*</nowiki>bacteria<nowiki>*</nowiki> | ||
| + | |align = "center"|360711 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''12''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene<nowiki>*</nowiki> OR ((C2 OR "C.sub.2") NEAR5 olefins)) | ||
| + | |align = "center"|410332 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''13''' | ||
| + | |Full patent spec. | ||
| + | |<nowiki>*</nowiki>methionine OR (acc ADJ1 synthase) OR "1-aminocyclopropane-1-carboxylate synthase" OR ACCsynthase OR (<nowiki>*</nowiki>2aminocyclopropane<nowiki>*</nowiki>3carboxylate ADJ1 synthase) | ||
| + | |align = "center"|60755 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''14''' | ||
| + | | | ||
| + | |align = "center"| C12N OR C12P | ||
| + | |align = "center"|570454 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''15''' | ||
| + | |Combined Query | ||
| + | |align = "center"|11 AND 12 AND 13 | ||
| + | |align = "center"|237 ('''131 Unique hits''') | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''19''' | ||
| + | |align = "center" rowspan = "5"|'''Ethylene oxide from ethylene''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene<nowiki>*</nowiki> OR ((C2 OR "C.sub.2") NEAR5 olefins)) | ||
| + | |align = "center"|410332 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''20''' | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"|oxidation OR produc<nowiki>*</nowiki> OR prepar<nowiki>*</nowiki> OR manufactur<nowiki>*</nowiki> OR convers<nowiki>*</nowiki> OR synthsi<nowiki>*</nowiki> | ||
| + | |align = "center"| 8311728 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''21''' | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"|((Ethylene ADJ1 oxide) OR Epoxyaethan OR Epoxyethane OR Oxidoethane OR (Ethene ADJ1 oxide) OR Oxirane) | ||
| + | |align = "center"| 59403 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''22''' | ||
| + | |Any classification | ||
| + | |align = "center"|B01J002104 OR B01J002108 OR B01J002304 OR B01J002350 OR C07D30103 OR B01D005304 OR B01J002112 OR B01J002712 OR B01J002708 OR B01J002710 OR B01J002709 OR B01J002308 OR B01J002368 OR B01J0027055 OR B01J002324 OR 00B01J002336 OR B01J002330 OR B01J002300 OR 00B01J2370 OR C07D30110 OR B01J002348 OR B01J2702 OR B01J2714 OR B01J2706 OR C07D30304 OR B01J00236200 OR B01J002366 OR B01D005304 OR B09J002104 OR B09J002108 OR B01J002396 OR B01J003102 OR B01J003100 OR B01J002118 OR B01J00208 OR B09J002112 OR 00B09J002350 OR B01J002302 OR B01J002303 OR B01J002720 OR B01J00231000B01J002378 OR C07D30132 OR B01J002106 OR B01J002396 OR C07D030132 OR C07D030100 OR C07C002910 OR C07C002910 OR C07C002900 OR C07C002909 OR C07C002700 OR C07D030106 OR B01J0027199 OR B01J002714 OR C07C005700 | ||
| + | |align = "center"| 195483 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''23''' | ||
| + | |Combined query | ||
| + | |align = "center"|19 AND 20 AND 21 AND 22 | ||
| + | |align = "center"| 2144 '''(1167 unique records)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|''' ''' | ||
| + | |align = "center" bgcolor = "#99CCFF"| | ||
| + | |bgcolor = "#99CCFF"| | ||
| + | |align = "center" bgcolor = "#99CCFF"| | ||
| + | |align = "center" bgcolor = "#99CCFF"| | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''24''' | ||
| + | |align = "center" rowspan = "3"|'''Ethylene glycol from ethylene oxide''' | ||
| + | |Ethylene glycol manufacture | ||
| + | |align = "center"|(<nowiki>*</nowiki>4Dihydroxyethane OR <nowiki>*</nowiki>4Ethandiol OR <nowiki>*</nowiki>4Ethanediol OR GLYCOL OR <nowiki>*</nowiki>4Ethylene<nowiki>*</nowiki>1Glycol OR <nowiki>*</nowiki>2Hydroxyethanol OR <nowiki>*</nowiki>2Hydroyethanol OR Aethylenglykol OR Athylenglykol OR Athylenglykol OR Diphyl OR Dowtherm<nowiki>*</nowiki>3 OR Ethane<nowiki>*</nowiki>4diol OR ethanediol OR Ethylene<nowiki>*</nowiki>1alcohol OR Ethylene<nowiki>*</nowiki>1Glycol OR Ethylene<nowiki>*</nowiki>1glycol OR Fridex OR Glycol<nowiki>*</nowiki>1alcohol OR Lutrol<nowiki>*</nowiki>19 OR MEG OR Monoethylene<nowiki>*</nowiki>glycol OR Therminol <nowiki>*</nowiki>VP OR (ethylene ADJ1 glycol<nowiki>*</nowiki>) OR ethyleneglycol<nowiki>*</nowiki>)SAME(produc<nowiki>*</nowiki> OR prepar<nowiki>*</nowiki> OR manufact<nowiki>*</nowiki> OR synthe<nowiki>*</nowiki> OR conver<nowiki>*</nowiki>)) | ||
| + | |align = "center"|75928 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''25''' | ||
| + | |Any classification | ||
| + | |align = "center"|C07D030108 OR C07B006100 OR C07C002948 OR C07C002950 OR C07C003120 OR C07D030103 OR C07D030104 OR C07D030122 OR C07D030300 OR C07D030304 OR C07B006100 OR C07C002900 OR C07C003100 OR C07D030100 OR C07D030300 OR C07C002910 OR C07C002909 OR C07C002700 OR C07C002726 OR C07C002974 OR C07C003102 OR C07C003300 OR C07C003120 OR C07C003118 OR C08G006526 OR B01J003108 OR C07C002980 OR C07C002976 OR C07C004103 OR C07C004310 OR C07C004311 OR C07D030110 OR C07C004100 OR C07C006900 OR C07C006800 OR C07C002900 OR C07C006806 OR C07C0029128 OR C07C002728 OR C07C002702 OR C07C002980 OR C07C002909 OR B01J003106 OR C07C003120 OR C07D031736 OR C07D031738 OR C07D031700 | ||
| + | |align = "center"|290188 (hits Unique hits) | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''26''' | ||
| + | |Combined Query | ||
| + | |align = "center"|21 AND 24 AND 25 | ||
| + | |align = "center"|858(591 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|''' ''' | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "4"| | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''27''' | ||
| + | |align = "center" rowspan = "4"|'''Preparation of benzeneamide from benzene nitrile''' | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"|(benzonitrile OR (benzene ADJ1 nitrile)OR cyanobenzene OR (cyno ADJ1 benzene ) OR (Benzene ADJ1 cyanide) OR (phenyl ADJ1 cyanide)OR (Benzene ADJ1 carbo ADJ1 nitrile) OR (Benzoic ADJ1 acid ADJ1 nitrile)) | ||
| + | |align = "center"|41789 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''28''' | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"|(microorganism<nowiki>*</nowiki> OR microb<nowiki>*</nowiki> OR bacteria OR yeast OR fungi OR bacteri<nowiki>*</nowiki> OR bioproduction OR biosynthesi<nowiki>*</nowiki> ) | ||
| + | |align = "center"| 339673 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''29''' | ||
| + | |Full patent spec. | ||
| + | |align = "center"| (benzene ADJ1 amide) OR (phenyl ADJ1 amide)OR amide) | ||
| + | |align = "center"| 143811 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''30''' | ||
| + | |Combined query | ||
| + | |align = "center"|27 AND 28 AND 29 | ||
| + | |align = "center"|305'''(162 unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''15''' | ||
| + | |align = "center" colspan = "2"|'''All Combined''' | ||
| + | |align = "center"|4 OR 10 OR 15 OR 23 OR 26 OR 30 | ||
| + | |align = "center"|5674''' (3644 unique records)''' | ||
| + | |- | ||
| + | |align = "center" colspan = "5"|Relevancy-25% | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFFF" colspan = "5"|'''Query 2''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''S.No''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search string''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Hits''' | ||
| + | |- | ||
| + | |align = "right"|1 | ||
| + | |align = "center" rowspan = "4"|'''Preparation of ethanol from fermentation''' | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"| ferment<nowiki>*</nowiki> OR zymology OR zymoptic | ||
| + | |align = "center"|41789 hits | ||
| + | |- | ||
| + | |align = "right"|2 | ||
| + | |Claims, Title or Abstract | ||
| + | |align = "center"|(Hydroxyethane OR (Methyl ADJ1 carbinol) OR (Pure ADJ1 alcohol) OR (Grain ADJ1 alcohol) OR (Ethyl ADJ1 alcohol) OR Ethanol<nowiki>*</nowiki> OR bioethanol<nowiki>*</nowiki> OR (fermentation ADJ1 alcohol) OR methylcarbinol) | ||
| + | |align = "center"| 339673 hits | ||
| + | |- | ||
| + | | | ||
| + | |Any classification | ||
| + | |align = "center"| C12P OR C12N OR C12F OR C07C OR C07D | ||
| + | |align = "center"|305'''(162 unique hits)''' | ||
| + | |- | ||
| + | |align = "right"|4 | ||
| + | |Combined query | ||
| + | |align = "center"|1 AND 2 AND 3 | ||
| + | |align = "center"|3905'''(2207 unique hits)''' | ||
| + | |- | ||
| + | |align = "center" colspan = "5"|'''Relevancy-50%''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFFF" colspan = "5"|'''Final Query''' | ||
| + | |- | ||
| + | |'''Final Query''' | ||
| + | | | ||
| + | |align = "center"|Combined query | ||
| + | |align = "center"|'''Query1 <nowiki>+</nowiki> Query 2''' | ||
| + | |align = "center"|9669('''5847 unique hits)''' | ||
| + | |- | ||
| + | |align = "center" colspan = "5"|'''Relevancy-30%''' | ||
| + | |- | ||
| + | |} | ||
| − | |||
| − | === | + | Note:The above mentioned benzamide is converted to benzoic acid which is then converted to terephthalic acid. |
| − | [ | + | * '''Benzoic acid to terepthalic acid''' [http://www.google.com/patents?id=aHRyAAAAEBAJ&printsec=abstract&zoom=4&source=gbs_summary_r&cad=0_0 US2823230] |
| + | *'''Benzene amide to benzoic acid''' [http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5932454.PN.&OS=PN/5932454&RS=PN/5932454 US5932454A] | ||
| − | == | + | === New search stratergy=== |
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | |
| + | |align = "center" bgcolor = "#99CCFF"|'''S.No''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search string''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Hits''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFFF" colspan = "5"|'''Query 1''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''1''' | ||
| + | |align = "center"|'''Bio-Production of Terephthalic acid''' | ||
| + | |Full patent spec. | ||
| + | |(((terephthalic OR Terepthalic) NEAR4 (produce<nowiki>*</nowiki> OR production OR conver<nowiki>*</nowiki> OR synthes<nowiki>*</nowiki> OR biotransformation)) NEAR10 ((plant NEAR5 source) OR biological OR glucose OR fructose<nowiki>*</nowiki> OR sucrose OR starch OR cellulose OR biomass OR waste<nowiki>*</nowiki> OR biotransformation OR feedstock)) | ||
| + | |align = "center"| 152 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''2''' | ||
| + | |align = "center" rowspan = "3"|'''Production of Terephthalic acid from 2,5-furandicarboxylate''' | ||
| + | |Full patent spec. | ||
| + | |(<nowiki>*</nowiki>4Benzenecarboxylic OR <nowiki>*</nowiki>4Benzenedicarboxylic OR Benzene<nowiki>*</nowiki>2para<nowiki>*</nowiki>2dicarboxylic OR benzene<nowiki>*</nowiki>5dicarboxylic OR Benzene<nowiki>*</nowiki>p<nowiki>*</nowiki>dicarboxylic OR p<nowiki>*</nowiki>Benzenedicarboxylic OR p<nowiki>*</nowiki>Carboxybenzoic OR p<nowiki>*</nowiki>Phthalic OR Para<nowiki>*</nowiki>Phthalic OR (Terephthalic ADJ1 acid) OR Acide<nowiki>*</nowiki>2terephtalique OR Kyselina<nowiki>*</nowiki>2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | ||
| + | |align = "center"|104258 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''3''' | ||
| + | |Full patent spec. | ||
| + | |"2,5-furandicarboxylate" OR <nowiki>*</nowiki>furandicarboxylate OR (furane ADJ1 dicarboxylate) OR furfurandicarboxylate OR (oxole ADJ1 dicarboxylate)OR (divinyl ADJ1 oxide ADJ1 dicarboxylate) | ||
| + | |align = "center"|174 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''4''' | ||
| + | |Combined Query | ||
| + | |align = "center"|2 AND 3 | ||
| + | |align = "center"|20 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''5''' | ||
| + | |align = "center" rowspan = "4"|'''Bio-Production of Terephthalic acid from Xylene''' | ||
| + | |Full patent spec. | ||
| + | |(((<nowiki>*</nowiki>4Benzenecarboxylic OR <nowiki>*</nowiki>4Benzenedicarboxylic OR Benzene<nowiki>*</nowiki>2para<nowiki>*</nowiki>2dicarboxylic OR benzene<nowiki>*</nowiki>5dicarboxylic OR Benzene<nowiki>*</nowiki>p<nowiki>*</nowiki>dicarboxylic OR p<nowiki>*</nowiki>Benzenedicarboxylic OR p<nowiki>*</nowiki>Carboxybenzoic OR p<nowiki>*</nowiki>Phthalic OR Para<nowiki>*</nowiki>Phthalic OR Terephthalic) ADJ acid) OR Acide<nowiki>*</nowiki>2terephtalique OR Kyselina<nowiki>*</nowiki>2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | ||
| + | |align = "center"| 102948 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''6''' | ||
| + | |Claims, Title or Abstract | ||
| + | |((bio<nowiki>*</nowiki> OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur<nowiki>*</nowiki>3 OR transformation OR transform OR conversion OR cataly<nowiki>*</nowiki>3 OR <nowiki>*</nowiki>synthe<nowiki>*</nowiki>)) | ||
| + | |align = "center"|360711 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''7''' | ||
| + | |Full patent spec. | ||
| + | | "1,4-dimethylbenzene" OR "p-methyltoluene" OR "1,4-xylene" OR <nowiki>*</nowiki>5dimethylbenzene OR <nowiki>*</nowiki>5xylene OR <nowiki>*</nowiki>3methyltoluene | ||
| + | |align = "center"|507183 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''8''' | ||
| + | |Combined Query | ||
| + | |align = "center"|5 AND 6 AND 7 | ||
| + | |align = "center"|25'''(20 Unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''9''' | ||
| + | |align = "center" colspan = "2"|'''Combined Query''' | ||
| + | |align = "center"|1 OR 4OR 8 | ||
| + | |align = "center"|189 ('''101 Unique Hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" colspan = "5"|Relevancy-10% | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFFF" colspan = "5"|'''Query 2''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''S.No''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search string''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Hits''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''10''' | ||
| + | |align = "center"|'''Production of Ethylene through Biological process''' | ||
| + | |Claims, Title or Abstract | ||
| + | |((bio OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur<nowiki>*</nowiki>3 OR transformation OR transform OR conversation OR cataly<nowiki>*</nowiki>3)) OR ferment<nowiki>*</nowiki> OR microorganism<nowiki>*</nowiki> OR microb<nowiki>*</nowiki> OR <nowiki>*</nowiki>bacteria<nowiki>*</nowiki> | ||
| + | |align = "center"|360711 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''11''' | ||
| + | |align = "center"|''' ''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene<nowiki>*</nowiki> OR ((C2 OR "C.sub.2") NEAR5 olefins)) | ||
| + | |align = "center"|410332 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''12''' | ||
| + | |align = "center"|''' ''' | ||
| + | |Full patent spec. | ||
| + | |<nowiki>*</nowiki>methionine OR (acc ADJ1 synthase) OR "1-aminocyclopropane-1-carboxylate synthase" OR ACCsynthase OR (<nowiki>*</nowiki>2aminocyclopropane<nowiki>*</nowiki>3carboxylate ADJ1 synthase) | ||
| + | |align = "center"|60755 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''13''' | ||
| + | |align = "center"|''' ''' | ||
| + | | | ||
| + | |align = "center"| C12N OR C12P | ||
| + | |align = "center"|570454 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''14''' | ||
| + | |align = "center"|''' ''' | ||
| + | |Combined Query | ||
| + | |align = "center"|11 AND 12 AND 13 | ||
| + | |align = "center"|331 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|''' ''' | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "4"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''15''' | ||
| + | |align = "center" rowspan = "6"|'''Production of Ethylene from Ethanol''' | ||
| + | |Claims, Title or Abstract | ||
| + | |Ethanol OR (ethyl ADJ1 alcohol) OR (grain ADJ1 alcohol) | ||
| + | |align = "center"|134288 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''16''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene<nowiki>*</nowiki> OR ((C2 OR "C.sub.2") NEAR5 olefins)) | ||
| + | |align = "center"|410332 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''17''' | ||
| + | |Claims, Title or Abstract | ||
| + | |produc<nowiki>*</nowiki>5 OR conver<nowiki>*</nowiki>5 OR manufactur<nowiki>*</nowiki>3 OR synthes<nowiki>*</nowiki>6 OR process OR method | ||
| + | |align = "center"|13960381 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''18''' | ||
| + | |Claims, Title or Abstract | ||
| + | |(dehydrat<nowiki>*</nowiki> OR (water ADJ1 removal) OR hypohydrat<nowiki>*</nowiki> OR cataly<nowiki>*</nowiki> OR zeolite<nowiki>*</nowiki> OR silica OR alumina OR (trifluoromethanesulfonic ADJ1 acid) OR evaporat<nowiki>*</nowiki> OR desiccation OR drying OR trifluoromethanesulfonicacid OR (water NEAR5 remov<nowiki>*</nowiki>)) | ||
| + | |align = "center"|1643133 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''19''' | ||
| + | |Any Classification | ||
| + | |align = "center"|C07C OR B01D | ||
| + | |align = "center"|1267051 hits | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''20''' | ||
| + | |Combined Query | ||
| + | |align = "center"|15 OR 16 OR 17 OR 18 OR 19 | ||
| + | |align = "center"|2606''' (1863 Unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''20''' | ||
| + | |align = "center" colspan = "2"|'''Final Query''' | ||
| + | |align = "center"|14 OR 20 | ||
| + | |align = "center"|2936''' (2046 Unique hits)''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''20''' | ||
| + | |align = "center" colspan = "4"|Relevency-20% | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "5"|''' ''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''21''' | ||
| + | |align = "center" colspan = "2"|'''Final Query''' | ||
| + | |align = "center"|'''Query 1<nowiki>+</nowiki>Query2''' | ||
| + | |align = "center"|3123('''2145 unique hits''') | ||
| + | |- | ||
| + | |align = "center" colspan = "5"|Relevancy-15% | ||
| + | |- | ||
| + | |} | ||
| − | + | ---- | |
| − | === | + | =='''IP Analysis for conversion of ethanol into ethylene '''== |
| − | + | ==='''IP Activity'''=== | |
| − | + | [[Image:Ip Activity.jpg|center|thumb|700px| '''IP Activity''']] | |
| − | + | ==='''Top Assignee for patents related to conversion of Ethanol to Ethylene'''=== | |
| + | [[Image:Top assignee2.jpg|center|thumb|800px| '''Top Assignees''']] | ||
| − | |||
| − | |||
| − | + | ==Patent Analysis== | |
| − | + | #'''Click here for [[Media:Analysis sheet ethanol dehydration.xls| Patent Analysis Sheet]]''' | |
| − | + | ==Dashboard link== | |
| − | + | {|border="3" cellspacing="2" cellpadding="4" | |
| + | |- | ||
| + | |[https://www.dolcera.com/auth/dashboard/dashboard.php?workfile_id=561 '''Dashboard'''] | ||
| + | |- | ||
| + | |} | ||
| − | + | ---- | |
| − | === | + | == '''IP Analysis for patents related to biological production of 5-hydroxymethyl furfural '''== |
| + | ==='''IP Activity'''=== | ||
| − | + | [[Image:IP Activit.jpg|center|thumb|800px|'''Top Assignee''']] | |
| − | + | ==='''Top Assignee for patents related to biological production of 5-hydroxymethyl furfural'''=== | |
| − | + | [[Image:Top assigne1.jpg|center|thumb|800px| '''Top Assignees''']] | |
| − | + | ||
| − | + | ==Patent Analysis== | |
| − | + | ||
| − | + | #'''Click here for [[Media:Analysis sheet ethanol dehydration.xls| Patent Analysis Sheet]]''' | |
| − | [[ | + | |
| − | === | + | ==Dashboard link== |
| + | {|border="3" cellspacing="2" cellpadding="4" | ||
| + | |- | ||
| + | |[https://www.dolcera.com/auth/dashboard/dashboard.php?workfile_id=566 '''Dashboard'''] | ||
| + | |- | ||
| + | |} | ||
| + | ---- | ||
| − | + | == '''IP Analysis for conversion of ethanol into ethylene glycol and terephthalic acid '''== | |
| + | ==='''IP Activity'''=== | ||
| − | + | [[Image:IP Activiy.jpg|center|thumb|800px|'''Top Assignee''']] | |
| − | + | ||
| − | + | ==='''Top Assignee for patents related to conversion of Ethanol to ethylene glycol and terephthalic acid'''=== | |
| − | + | ||
| − | + | [[Image:Top assign3.jpg|center|thumb|800px| '''Top Assignees''']] | |
| − | [[Image: | + | |
| − | + | ==Patent Analysis== | |
| − | + | #'''Click here for [[Media:Analysis sheet ethanol dehydration.xls| Patent Analysis Sheet]]''' | |
| − | + | ==Dashboard link== | |
| − | + | {|border="3" cellspacing="2" cellpadding="4" | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | == | + | |
| − | + | ||
| − | + | ||
| − | {|border=" | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | ''' | + | |[https://www.dolcera.com/auth/dashboard/dashboard.php?workfile_id=567 '''Dashboard'''] |
|- | |- | ||
| − | |||
|} | |} | ||
Latest revision as of 05:52, 27 July 2015
PET (Polyethyeleneterephthalate) is used in a variety of applications, especially in the making of durable bottles. There is a significant increase in interest of late in producing PET from renewable sources.
This report describes the various techniques being used in the production of PET from sources such as cellulose. The report covers:
- Various pathways for production of PET from renewable sources
- All the catalysts (e.g. Hydrogen, Sodium, etc.) and techniques (e.g. catalytic dehydration) used in production of each of the intermediate compounds (e.g. ethylene glycol)
- Search strategies for patents for the various pathways and methods for production of ethylene glycol and dimethyl terephthalate, which are then used to produce PET
- Classification and in-depth analysis of the patents
- Presentation of results of the patent analysis on the Dolcera dashboard
Contents
- 1 Pathways for production of Polyethyeleneterephthalate
- 1.1 Polyethylene terephthalate
- 1.2 Ethylene glycol
- 1.3 Terephthalic acid
- 1.4 Search Strategy
- 1.5 IP Analysis for conversion of ethanol into ethylene
- 1.6 Patent Analysis
- 1.7 Dashboard link
- 1.8 IP Analysis for patents related to biological production of 5-hydroxymethyl furfural
- 1.9 Patent Analysis
- 1.10 Dashboard link
- 1.11 IP Analysis for conversion of ethanol into ethylene glycol and terephthalic acid
- 1.12 Patent Analysis
- 1.13 Dashboard link
Pathways for production of Polyethyeleneterephthalate
Polyethylene terephthalate
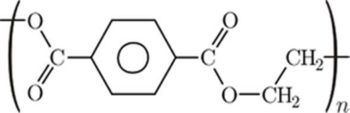
- Polyethylene terephthalate( (C10H8O4)n) (sometimes written poly(ethylene terephthalate)), is also abbreviated as PET, PETE, or PETP or PET-P)
- Some of the trade names of PET products are Dacron, Diolen, Tergal, Terylene, and Trevira fibers, Cleartuf, Eastman PET and Polyclear bottle resins, Hostaphan, Melinex, and Mylar films, and Arnite, Ertalyte, Impet, Rynite and Valox injection molding resins.
- The materials that can be prepared using PET are bottles, tapes, films or pulled into fibers that are pressed into meshes or woven into fabrics.
Preparation of Polyethyleneterephthalate
- Polyethyleneterephthalate is prepared by reacting Dimethylterephthalate with Ethylene glycol in the presence of a catalyst. Dimethylterephthalate is obtained by treating Terephthalic acid with methanol. The catalyst is a salt of a metal like calcium, barium, cadmium, cobalt, lead, manganese, tin, zinc and chromium and a material selected from the group consisting of hydroxybenzophenone carboxylic acids and hydroxyacetophenone carboxylic acids. Source
(Terephthalic acid) + 2CH3OH → (Dimethylterephthalate)
(Dimethylterephthalate) + CH2(OH)CH2(OH) + Catalyst → (Polyethyleneterephthalate)
Ethylene glycol
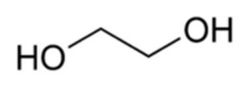
- Ethylene glycol(C2H4(OH)2), (monoethylene glycol (MEG), 1,2-ethanediol, IUPAC name: ethane-1,2- diol,)
- Ethylene glycol is an odorless, colorless, syrupy, sweet tasting, toxic liquid.
- It is used as a heat transfer chemical i.e., as a coolant. Due to its low freezing point it is used as a deicing fluid for windshields and aircraft.
- Ethylene glycol is widely used to inhibit the formation of natural gas clathrates in long multiphase pipelines. Then ethylene glycol can be recovered from the natural gas and reused as an inhibitor after purification treatment that removes water and inorganic salts.
- Ethylene glycol has become increasingly important in the plastics industry for the manufacture of polyester fibers and resins, including polyethylene terephthalate, which is used to make plastic bottles for soft drinks. The antifreeze capabilities of ethylene glycol have made it an important component of vitrification mixtures for low-temperature preservation of biological tissues and organs.
- Ethylene glycol may also be used as a protecting group for carbonyl groups in organic synthesis.
- Ethylene glycol's high boiling point and affinity for water makes it an ideal desiccant for natural gas production.
Preparation of Ethylene Glycol
Ethylene glycol is prepared in the following ways:
1. From ethylene: Ethylene is oxidized to ethylene oxide. Ethylene oxide upon hydrolysis ethylene glycol is prepared.
7 H2C=CH2 + 6 O2 → 6 C2H4O + 2 CO2 + 2 H2O
C2H4O + H2O → HOCH2CH2OH
2. Ethylene glycol is prepared by reacting formaldehyde with methanol in the presence of organic peroxide(ditertiarybutylperoxide) Source
CH3OH + HCHO + (CH3)3OOC(CH3)3→ CH2(OH)CH2(OH)
Note: In the above two preparation methods for ethylene glycol we have used ethylene, methanol, formaldehyde. The following are their preparations:
Ethylene preparation:
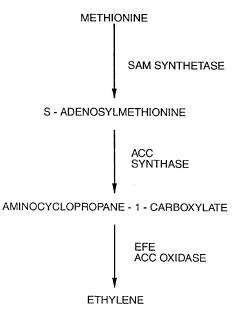
1. Ethylene is prepared biologically from methionine by using enzymes.Source
2. Ethanol upon dehydrating in the presence of catalyst ethylene is prepared.Source
C2H5OH + Al2O3(Catalyst) → H2C=CH2 + H2O
Methanol preparation: Biological process
Methanol is prepared through fermentation process. Vegetable materials are fermented anerobically in the presence of Zymomonas mobilis bacteria. In this process ethanol and methanol are the products. Methanol is separated from ethanol by distillation. Source
Formaldehyde preparation:
1. Formaldehyde is prepared enzymatically. In this process the lower alkyl alcohols are converted into lower aldehydes by using oxidase enzymes. Source
2. Formaldehyde is prepared by dehydrogenation of methanol using a catalyst. The catalyst is silver or silver/gold alloy on an inert hard, nonporous support. Source
CH3OH + Ag (or Ag/Au alloy)(Catalyst) → HCHO + H2
Ethanol preparation:
Ethanol is prepared by fermentation process.
Terephthalic acid
- Terephthalic acid(C6H4(CO2H)2) is a colourless solid, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually. It is one of three isomeric phthalic acids.
- Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET) Source
Preparation of Terephthalic acid
- Biological preparation of Terephthalic acid Source
Preparation of p-Xylene:
p-Xylene is prepared from toluene in the presence of a catalyst. This is a methylation reaction. The methylating agents are methanol, methyl chloride, methyl bromide, dimethylether or dimethylsulfide. The catalyst used is ZSM-23 zeolite material. Here this catalyst is used for attacking the methyl group in the para position of the toluene. Source
Toluene + Methylating agent + ZSM-23 Zeolite(Catalyst)→ P-xylene
Preparation of Toluene:
1. Toluene is an aromatic extract from the tropical colombian tree Myroxylon Balsamum Source
2. Toluene is prepared from wastes like old trees, cable wastes, polyethylene waster and polypropylene wastes. In this process the waste products are pulverised and heated to a temperature range of 150˚C to 500˚C at a pressure of 20 to 300 bar Source
Note-Today most of the ethylene manufacturers are producing ethylene through petroleum cracking.
Search Strategy
- Database - Micropat
- We conducted search on concepts that have a biological method of preparation only.
- We have not conducted search on production of ethanol (from which we prepare ethylene) since that is not the focus of this project.
- NOTE: Explanation of Query Concepts
- Query 1 - All Biological Process
- Query 2 - Ethanol Fermentation
- Final Query - Query 1 + Query 2
Issue/Publication Years: 1836 - Date (9th June 2009)
| S.No | Concept | Scope | Search string | Hits |
| Query 1 | ||||
| 1 | Bio-Production of Terepthalic acid from Xylene | Full patent spec. | (((*4Benzenecarboxylic OR *4Benzenedicarboxylic OR Benzene*2para*2dicarboxylic OR benzene*5dicarboxylic OR Benzene*p*dicarboxylic OR p*Benzenedicarboxylic OR p*Carboxybenzoic OR p*Phthalic OR Para*Phthalic OR Terephthalic) ADJ acid) OR Acide*2terephtalique OR Kyselina*2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | 102948 hits |
| 2 | Claims, Title or Abstract | ((bio* OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur*3 OR transformation OR transform OR conversion OR cataly*3 OR *synthe*)) | 360711 hits | |
| 3 | Full patent spec. | "1,4-dimethylbenzene" OR "p-methyltoluene" OR "1,4-xylene" OR *5dimethylbenzene OR *5xylene OR *3methyltoluene | 507183 hits | |
| 4 | Combined Query | 1 AND 2 AND 3 | 25(20 Unique hits) | |
| 5 | Production of Ethylene from Ethanol | Claims, Title or Abstract | Ethanol OR (ethyl ADJ1 alcohol) OR (grain ADJ1 alcohol) | 134288 hits |
| 6 | Claims, Title or Abstract | (Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene* OR ((C2 OR "C.sub.2") NEAR5 olefins)) | 410332 hits | |
| 7 | Claims, Title or Abstract | produc*5 OR conver*5 OR manufactur*3 OR synthes*6 OR process OR method | 13960381 hits | |
| 8 | Claims, Title or Abstract | (dehydrat* OR (water ADJ1 removal) OR hypohydrat* OR cataly* OR zeolite* OR silica OR alumina OR (trifluoromethanesulfonic ADJ1 acid) OR evaporat* OR desiccation OR drying OR trifluoromethanesulfonicacid OR (water NEAR5 remov*)) | 1643133 hits | |
| 9 | Any Classification | C07C OR B01D | 1267051 hits | |
| 10 | Combined Query | 5 AND 6 AND 7 AND 8 AND 9 | 2606 (1863 Unique hits) | |
| 11 | Production of Ethylene through Biological process | Claims, Title or Abstract | ((bio OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur*3 OR transformation OR transform OR conversation OR cataly*3)) OR ferment* OR microorganism* OR microb* OR *bacteria* | 360711 hits |
| 12 | Claims, Title or Abstract | (Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene* OR ((C2 OR "C.sub.2") NEAR5 olefins)) | 410332 hits | |
| 13 | Full patent spec. | *methionine OR (acc ADJ1 synthase) OR "1-aminocyclopropane-1-carboxylate synthase" OR ACCsynthase OR (*2aminocyclopropane*3carboxylate ADJ1 synthase) | 60755 hits | |
| 14 | C12N OR C12P | 570454 | ||
| 15 | Combined Query | 11 AND 12 AND 13 | 237 (131 Unique hits) | |
| 19 | Ethylene oxide from ethylene | Claims, Title or Abstract | (Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene* OR ((C2 OR "C.sub.2") NEAR5 olefins)) | 410332 hits |
| 20 | Claims, Title or Abstract | oxidation OR produc* OR prepar* OR manufactur* OR convers* OR synthsi* | 8311728 hits | |
| 21 | Claims, Title or Abstract | ((Ethylene ADJ1 oxide) OR Epoxyaethan OR Epoxyethane OR Oxidoethane OR (Ethene ADJ1 oxide) OR Oxirane) | 59403 hits | |
| 22 | Any classification | B01J002104 OR B01J002108 OR B01J002304 OR B01J002350 OR C07D30103 OR B01D005304 OR B01J002112 OR B01J002712 OR B01J002708 OR B01J002710 OR B01J002709 OR B01J002308 OR B01J002368 OR B01J0027055 OR B01J002324 OR 00B01J002336 OR B01J002330 OR B01J002300 OR 00B01J2370 OR C07D30110 OR B01J002348 OR B01J2702 OR B01J2714 OR B01J2706 OR C07D30304 OR B01J00236200 OR B01J002366 OR B01D005304 OR B09J002104 OR B09J002108 OR B01J002396 OR B01J003102 OR B01J003100 OR B01J002118 OR B01J00208 OR B09J002112 OR 00B09J002350 OR B01J002302 OR B01J002303 OR B01J002720 OR B01J00231000B01J002378 OR C07D30132 OR B01J002106 OR B01J002396 OR C07D030132 OR C07D030100 OR C07C002910 OR C07C002910 OR C07C002900 OR C07C002909 OR C07C002700 OR C07D030106 OR B01J0027199 OR B01J002714 OR C07C005700 | 195483 hits | |
| 23 | Combined query | 19 AND 20 AND 21 AND 22 | 2144 (1167 unique records) | |
| 24 | Ethylene glycol from ethylene oxide | Ethylene glycol manufacture | (*4Dihydroxyethane OR *4Ethandiol OR *4Ethanediol OR GLYCOL OR *4Ethylene*1Glycol OR *2Hydroxyethanol OR *2Hydroyethanol OR Aethylenglykol OR Athylenglykol OR Athylenglykol OR Diphyl OR Dowtherm*3 OR Ethane*4diol OR ethanediol OR Ethylene*1alcohol OR Ethylene*1Glycol OR Ethylene*1glycol OR Fridex OR Glycol*1alcohol OR Lutrol*19 OR MEG OR Monoethylene*glycol OR Therminol *VP OR (ethylene ADJ1 glycol*) OR ethyleneglycol*)SAME(produc* OR prepar* OR manufact* OR synthe* OR conver*)) | 75928 hits |
| 25 | Any classification | C07D030108 OR C07B006100 OR C07C002948 OR C07C002950 OR C07C003120 OR C07D030103 OR C07D030104 OR C07D030122 OR C07D030300 OR C07D030304 OR C07B006100 OR C07C002900 OR C07C003100 OR C07D030100 OR C07D030300 OR C07C002910 OR C07C002909 OR C07C002700 OR C07C002726 OR C07C002974 OR C07C003102 OR C07C003300 OR C07C003120 OR C07C003118 OR C08G006526 OR B01J003108 OR C07C002980 OR C07C002976 OR C07C004103 OR C07C004310 OR C07C004311 OR C07D030110 OR C07C004100 OR C07C006900 OR C07C006800 OR C07C002900 OR C07C006806 OR C07C0029128 OR C07C002728 OR C07C002702 OR C07C002980 OR C07C002909 OR B01J003106 OR C07C003120 OR C07D031736 OR C07D031738 OR C07D031700 | 290188 (hits Unique hits) | |
| 26 | Combined Query | 21 AND 24 AND 25 | 858(591 | |
| 27 | Preparation of benzeneamide from benzene nitrile | Claims, Title or Abstract | (benzonitrile OR (benzene ADJ1 nitrile)OR cyanobenzene OR (cyno ADJ1 benzene ) OR (Benzene ADJ1 cyanide) OR (phenyl ADJ1 cyanide)OR (Benzene ADJ1 carbo ADJ1 nitrile) OR (Benzoic ADJ1 acid ADJ1 nitrile)) | 41789 hits |
| 28 | Claims, Title or Abstract | (microorganism* OR microb* OR bacteria OR yeast OR fungi OR bacteri* OR bioproduction OR biosynthesi* ) | 339673 hits | |
| 29 | Full patent spec. | (benzene ADJ1 amide) OR (phenyl ADJ1 amide)OR amide) | 143811 hits | |
| 30 | Combined query | 27 AND 28 AND 29 | 305(162 unique hits) | |
| 15 | All Combined | 4 OR 10 OR 15 OR 23 OR 26 OR 30 | 5674 (3644 unique records) | |
| Relevancy-25% | ||||
| Query 2 | ||||
| S.No | Concept | Scope | Search string | Hits |
| 1 | Preparation of ethanol from fermentation | Claims, Title or Abstract | ferment* OR zymology OR zymoptic | 41789 hits |
| 2 | Claims, Title or Abstract | (Hydroxyethane OR (Methyl ADJ1 carbinol) OR (Pure ADJ1 alcohol) OR (Grain ADJ1 alcohol) OR (Ethyl ADJ1 alcohol) OR Ethanol* OR bioethanol* OR (fermentation ADJ1 alcohol) OR methylcarbinol) | 339673 hits | |
| Any classification | C12P OR C12N OR C12F OR C07C OR C07D | 305(162 unique hits) | ||
| 4 | Combined query | 1 AND 2 AND 3 | 3905(2207 unique hits) | |
| Relevancy-50% | ||||
| Final Query | ||||
| Final Query | Combined query | Query1 + Query 2 | 9669(5847 unique hits) | |
| Relevancy-30% | ||||
Note:The above mentioned benzamide is converted to benzoic acid which is then converted to terephthalic acid.
- Benzoic acid to terepthalic acid US2823230
- Benzene amide to benzoic acid US5932454A
New search stratergy
| S.No | Concept | Scope | Search string | Hits |
| Query 1 | ||||
| 1 | Bio-Production of Terephthalic acid | Full patent spec. | (((terephthalic OR Terepthalic) NEAR4 (produce* OR production OR conver* OR synthes* OR biotransformation)) NEAR10 ((plant NEAR5 source) OR biological OR glucose OR fructose* OR sucrose OR starch OR cellulose OR biomass OR waste* OR biotransformation OR feedstock)) | 152 hits |
| 2 | Production of Terephthalic acid from 2,5-furandicarboxylate | Full patent spec. | (*4Benzenecarboxylic OR *4Benzenedicarboxylic OR Benzene*2para*2dicarboxylic OR benzene*5dicarboxylic OR Benzene*p*dicarboxylic OR p*Benzenedicarboxylic OR p*Carboxybenzoic OR p*Phthalic OR Para*Phthalic OR (Terephthalic ADJ1 acid) OR Acide*2terephtalique OR Kyselina*2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | 104258 hits |
| 3 | Full patent spec. | "2,5-furandicarboxylate" OR *furandicarboxylate OR (furane ADJ1 dicarboxylate) OR furfurandicarboxylate OR (oxole ADJ1 dicarboxylate)OR (divinyl ADJ1 oxide ADJ1 dicarboxylate) | 174 hits | |
| 4 | Combined Query | 2 AND 3 | 20 | |
| 5 | Bio-Production of Terephthalic acid from Xylene | Full patent spec. | (((*4Benzenecarboxylic OR *4Benzenedicarboxylic OR Benzene*2para*2dicarboxylic OR benzene*5dicarboxylic OR Benzene*p*dicarboxylic OR p*Benzenedicarboxylic OR p*Carboxybenzoic OR p*Phthalic OR Para*Phthalic OR Terephthalic) ADJ acid) OR Acide*2terephtalique OR Kyselina*2tereftalova OR Tephthol) OR (terepthalic ADJ1 acid) | 102948 hits |
| 6 | Claims, Title or Abstract | ((bio* OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur*3 OR transformation OR transform OR conversion OR cataly*3 OR *synthe*)) | 360711 hits | |
| 7 | Full patent spec. | "1,4-dimethylbenzene" OR "p-methyltoluene" OR "1,4-xylene" OR *5dimethylbenzene OR *5xylene OR *3methyltoluene | 507183 hits | |
| 8 | Combined Query | 5 AND 6 AND 7 | 25(20 Unique hits) | |
| 9 | Combined Query | 1 OR 4OR 8 | 189 (101 Unique Hits) | |
| Relevancy-10% | ||||
| Query 2 | ||||
| S.No | Concept | Scope | Search string | Hits |
| 10 | Production of Ethylene through Biological process | Claims, Title or Abstract | ((bio OR biological) SAME (prepare OR preparation OR extract OR extraction OR synthesis OR syntheses OR sinthisis OR synthesize OR production OR produce OR making OR manufactur*3 OR transformation OR transform OR conversation OR cataly*3)) OR ferment* OR microorganism* OR microb* OR *bacteria* | 360711 hits |
| 11 | Claims, Title or Abstract | (Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene* OR ((C2 OR "C.sub.2") NEAR5 olefins)) | 410332 hits | |
| 12 | Full patent spec. | *methionine OR (acc ADJ1 synthase) OR "1-aminocyclopropane-1-carboxylate synthase" OR ACCsynthase OR (*2aminocyclopropane*3carboxylate ADJ1 synthase) | 60755 hits | |
| 13 | C12N OR C12P | 570454 | ||
| 14 | Combined Query | 11 AND 12 AND 13 | 331 | |
| 15 | Production of Ethylene from Ethanol | Claims, Title or Abstract | Ethanol OR (ethyl ADJ1 alcohol) OR (grain ADJ1 alcohol) | 134288 hits |
| 16 | Claims, Title or Abstract | (Ethylene OR ethene OR acetene OR (bicarburetted ADJ1 hydrogen) OR elayl OR Etileno OR ethylene* OR ((C2 OR "C.sub.2") NEAR5 olefins)) | 410332 hits | |
| 17 | Claims, Title or Abstract | produc*5 OR conver*5 OR manufactur*3 OR synthes*6 OR process OR method | 13960381 hits | |
| 18 | Claims, Title or Abstract | (dehydrat* OR (water ADJ1 removal) OR hypohydrat* OR cataly* OR zeolite* OR silica OR alumina OR (trifluoromethanesulfonic ADJ1 acid) OR evaporat* OR desiccation OR drying OR trifluoromethanesulfonicacid OR (water NEAR5 remov*)) | 1643133 hits | |
| 19 | Any Classification | C07C OR B01D | 1267051 hits | |
| 20 | Combined Query | 15 OR 16 OR 17 OR 18 OR 19 | 2606 (1863 Unique hits) | |
| 20 | Final Query | 14 OR 20 | 2936 (2046 Unique hits) | |
| 20 | Relevency-20% | |||
| 21 | Final Query | Query 1+Query2 | 3123(2145 unique hits) | |
| Relevancy-15% | ||||
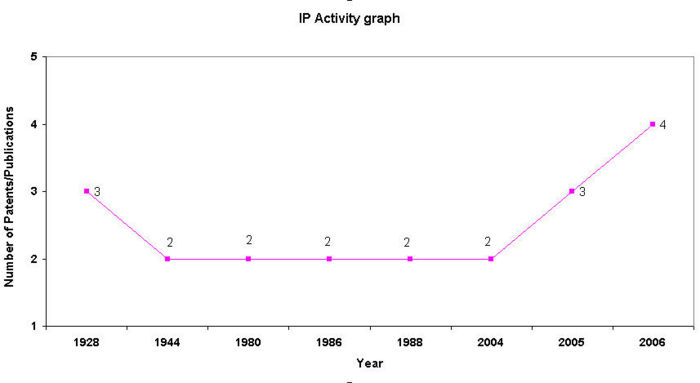
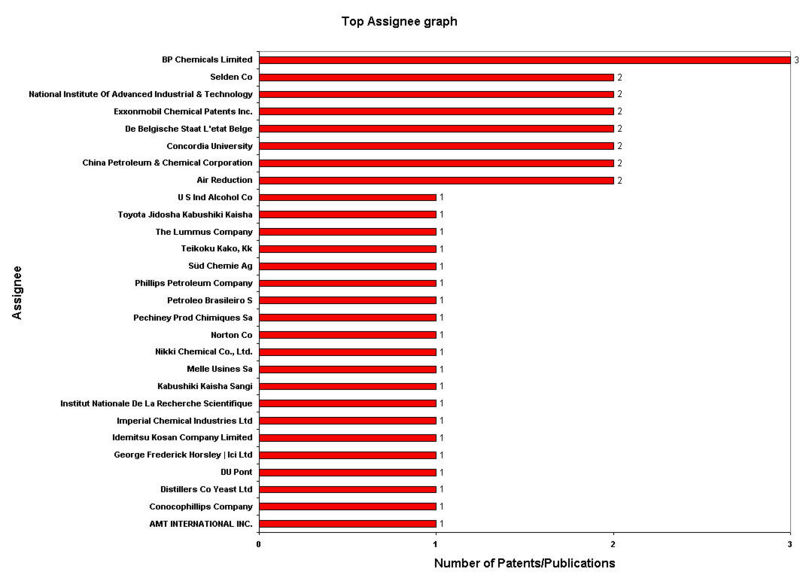
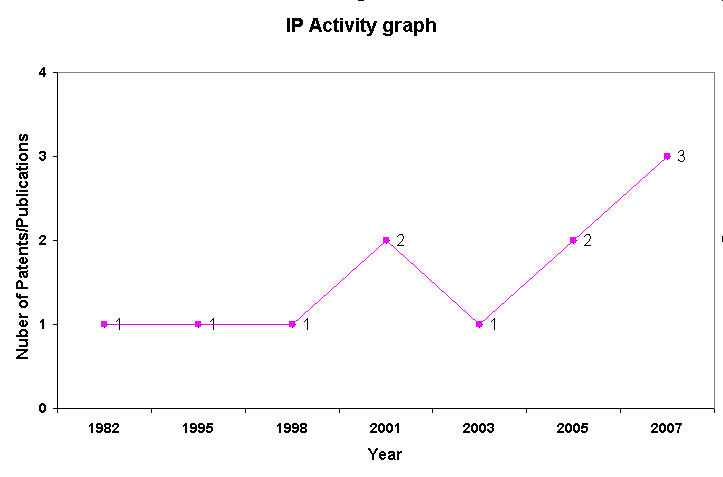
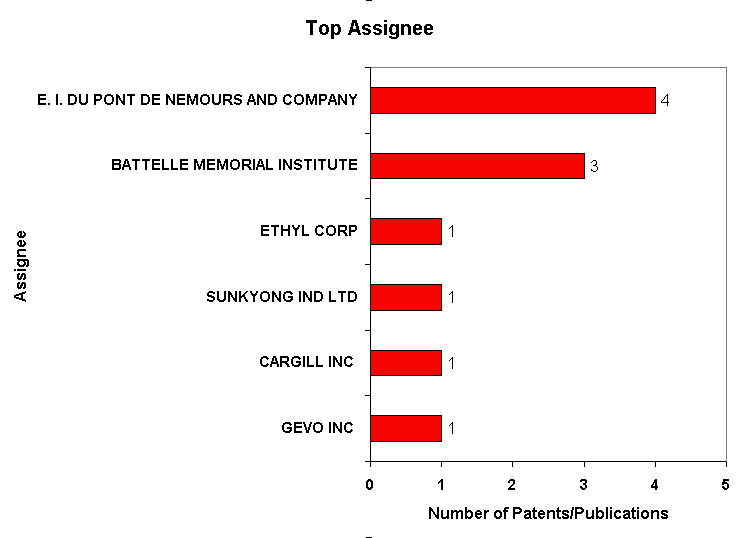
IP Analysis for conversion of ethanol into ethylene
IP Activity
Patent Analysis
- Click here for Patent Analysis Sheet
Dashboard link
| Dashboard |
IP Activity
Patent Analysis
- Click here for Patent Analysis Sheet
Dashboard link
| Dashboard |
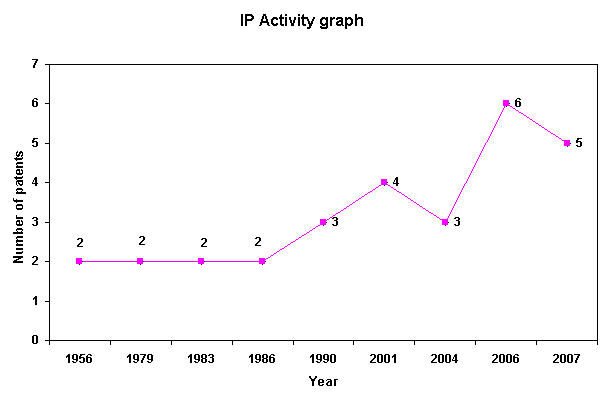
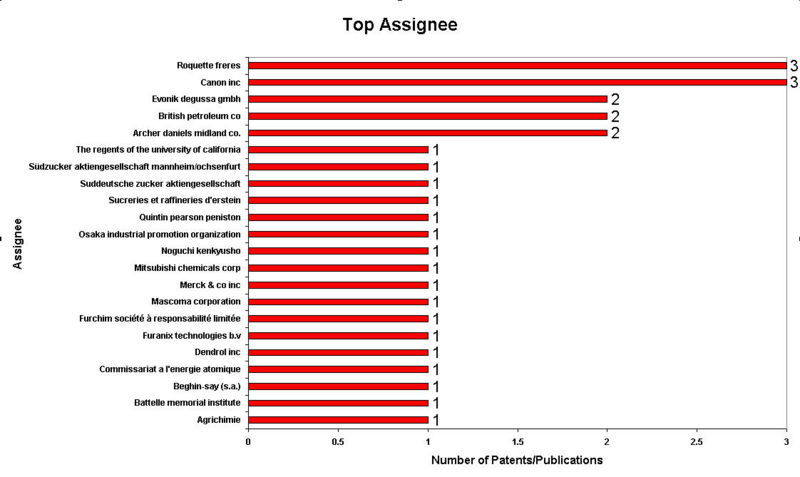
IP Analysis for conversion of ethanol into ethylene glycol and terephthalic acid
IP Activity
Patent Analysis
- Click here for Patent Analysis Sheet
Dashboard link
| Dashboard |